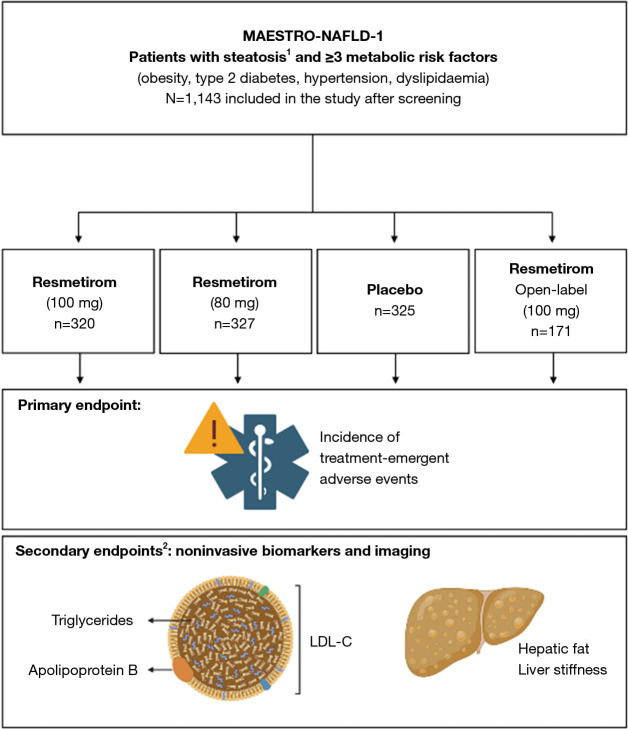
Figure 1.
Trial design of the MAESTRO-NAFLD-1 trial. The MAESTRO-NAFLD-1 (NCT04197479) trial [adapted from (7)] randomized patients to three double-blind arms. Treatment-emergent adverse events constituted the primary endpoint, metabolic and hepatic readouts were the secondary endpoints. Given numbers represent randomized subjects. Figure created with Biorender.com. 1, non-invasive testing for screening; 2, selected secondary outcomes are depicted. LDL-C, low-density lipoprotein-cholesterol.