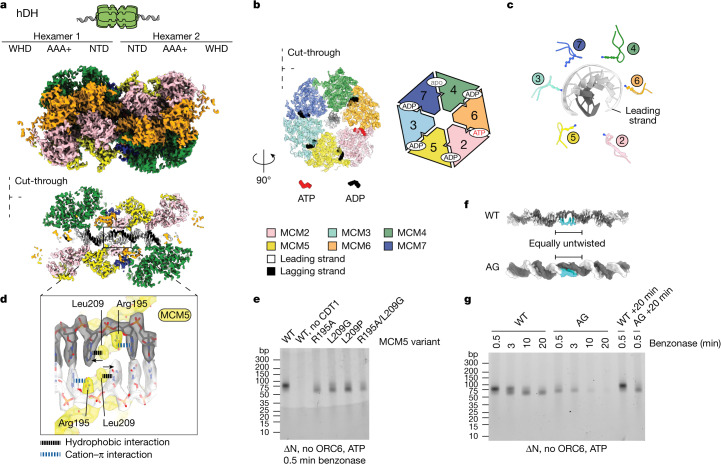
Fig. 2. hDH untwists and melts DNA.
a, Surface rendering and cut-through view of the hDH loaded onto duplex DNA. The double helix is untwisted and one base pair is broken at the dimerization interface. This distortion enables DNA to traverse a narrow passage created by two MCM5 zinc-finger domains. NTD, N-terminal domain; WHD, WH domain. b, View of C-terminal ATPases of one MCM ring. Nucleotides are shown in the ATPase sites between MCM subunits. c, PS1 pore loops form a staircase arrangement that follows the leading strand template. d, Zoomed-in view of the broken base pair stabilized by R195 and L209 of MCM5. e, Nuclease footprinting assay with wild-type (WT) and indicated mutants of MCM5. f, Comparison between duplex DNA in the wild-type MCM5 and MCM5(R195A/L209G) mutant (AG) double hexamer reveals a similar degree of DNA untwisting. g, Wild-type and AG mutant MCM were loaded on DNA and subjected to benzonase treatment lasting between 0.5 and 20 min. Controls were incubated for an additional 20 min followed by 0.5 min benzonase treatment.