FIGURE 1.
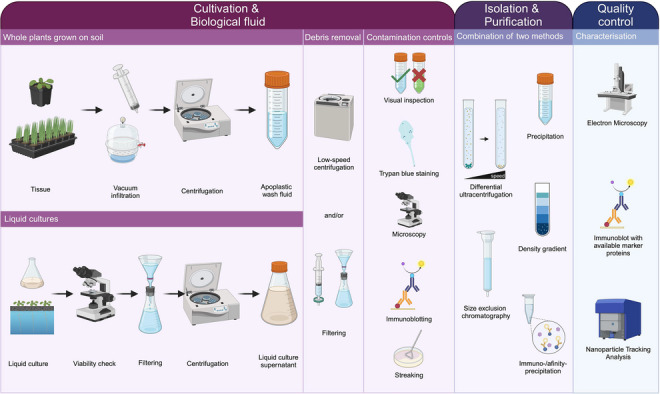
A workflow for designing EV isolation procedures from different sources in plant–microbe systems. The growth conditions of the source organism and the research question determine the downstream workflow for EV isolation. Cultivation and biological fluid: For plants grown on soil, the biological fluid, AWF, is commonly obtained by vacuum infiltration followed by very low‐speed centrifugation. Removal of cellular debris involves low‐speed centrifugation and filtering. Contamination controls for the tissue include visual inspection of the AWF colour (if applicable), viability stains (e.g., trypan blue), microscopy for organelle debris, and immunoblotting targeting intracellular proteins. When organisms are grown in liquid culture (e.g., microbes, plant cell cultures, hydroponic systems), the culture supernatant or medium serves as the biological fluid. For cell cultures, viability checks of source cells before harvesting the supernatant are advised. Removal of intact cells and larger debris involves filtering and centrifugation, followed by another step of low‐speed centrifugation and/or filtering for cell debris. Controls for contamination with live cells or cell debris are microscopy, immunoblotting with known non‐EV markers, and streaking of the cell‐free culture supernatant, where applicable. Isolation and purification of EVs can be achieved by precipitation via polymers, differential ultracentrifugation, density gradient centrifugation, size exclusion chromatography, or immuno‐/affinity‐purification. Details may vary based on research questions and the manageability of obtaining biological fluid. We refer to the MISEV 2023 guidelines for a detailed register of pros and cons for the different approaches (Welsh et al., 2024). Working with crude EV samples is acceptable for pilot studies, but purification with a second method exploiting different physical and biological properties should be performed for thorough examination. Quality control and initial characterization of isolated (and purified) EVs largely overlap. Two independent methods are recommended for EV characterization, with regular quality control suggested. Electron microscopy confirms the presence of typical structures, and nanoparticle tracking validates consistent particle size isolation, both independent of knowledge about biomarkers. If available, EVs can be tested for the presence of suitable marker proteins by immunoblotting. When EVs are isolated from unicellular organisms, streaking of the EV pellet aids in corroborating that no replicating entities are present. Created with BioRender.com. AWF, apoplastic wash fluid; EV, extracellular vesicles; MISEV, minimal information for studies of extracellular vesicles.
