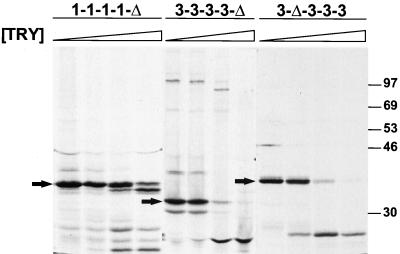
FIG. 4.
Effect of trypsin treatment on truncated ς1 proteins. 35S-labeled ς1 proteins were purified from insect cell lysates using antireovirus serum plus protein A-Sepharose, followed by treatment at 15°C with 0, 0.1, 1.0, or 10 μg of bovine trypsin per ml for 75 min. Reaction mixtures were heated at 100°C in protein sample buffer; digestion products were resolved in an SDS–10% polyacrylamide gel and visualized by autoradiography.  , 0 to 10 μg of trypsin [TRY] per ml. Positions of untreated ς1 deletion mutants are indicated by arrows. Positions of molecular weight standards (in kilodaltons) are shown.
, 0 to 10 μg of trypsin [TRY] per ml. Positions of untreated ς1 deletion mutants are indicated by arrows. Positions of molecular weight standards (in kilodaltons) are shown.