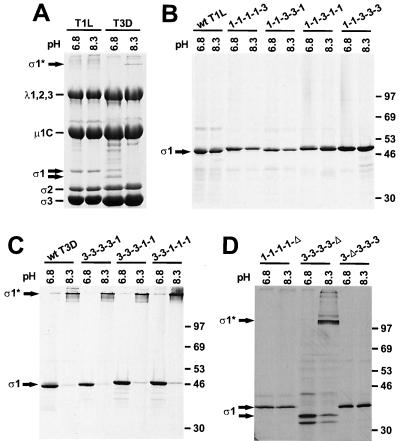
FIG. 5.
Oligomer stability of chimeric and truncated ς1 proteins. (A) 35S-labeled purified T1L and T3D virions were incubated at 100°C in pH 6.8 protein sample buffer or 60°C in pH 8.3 protein sample buffer for 10 min. Reaction products were resolved in an SDS–10% polyacrylamide gel and visualized by autoradiography. Viral structural proteins are labeled. (B and C) 35S-labeled full-length wt and chimeric ς1 proteins were purified from insect cell lysates using anti-ς1 MAb 5C6 or 9BG5 conjugated to Sepharose and treated as for panel A. Positions of molecular weight standards (in kilodaltons) are shown. (D) 35S-labeled truncated ς1 proteins were purified from insect cell lysates using MAb 9BG5 conjugated to Sepharose or antireovirus serum plus protein A-Sepharose and treated as for panel A. Positions of molecular weight standards (in kilodaltons) are shown. ς1*, bands corresponding to ς1 oligomers.