Abstract
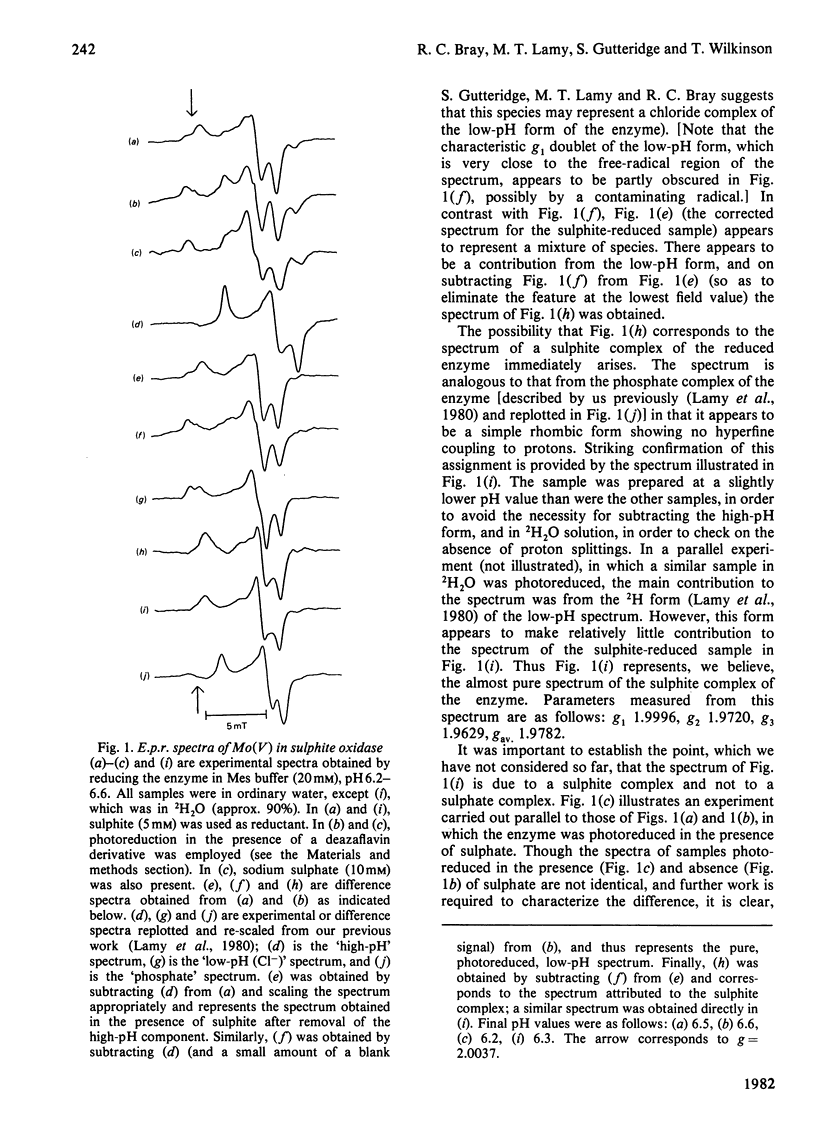
Reduction of sulphite oxidase by sulphite at low pH values in Mes (4-morpholine-ethanesulphonic acid) buffer gives rise to a new molybdenum(V) electron-paramagnetic-resonance spectrum different from that obtained by photoreduction of the enzyme in the same medium. The spectrum is attributed to a sulphite complex of the enzyme, showing g-values of about 2.000, 1.972 and 1.963. The complex is analogous to that with the inhibitor phosphate in that it gives rise to no observable hyperfine coupling of Mo(V) to exchangeable protons.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray R. C., Barber M. J., Lowe D. J. Electron-paramagnetic-resonance spectroscopy of complexes of xanthine oxidase with xanthine and uric acid. Biochem J. 1978 Jun 1;171(3):653–658. doi: 10.1042/bj1710653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Lamy M. T., Bray R. C. The nature of the phosphate inhibitor complex of sulphite oxidase from electron-paramagnetic-resonance studies using oxygen-17. Biochem J. 1980 Oct 1;191(1):285–288. doi: 10.1042/bj1910285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy M. T., Gutteridge S., Bary R. C. Electron-paramagnetic-resonance parameters of molybdenum(V) in sulphite oxidase from chicken liver. Biochem J. 1980 Feb 1;185(2):397–403. doi: 10.1042/bj1850397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Hemmerich P. A photochemical procedure for reduction of oxidation-reduction proteins employing deazariboflavin as catalyst. J Biol Chem. 1977 Aug 25;252(16):5612–5614. [PubMed] [Google Scholar]


