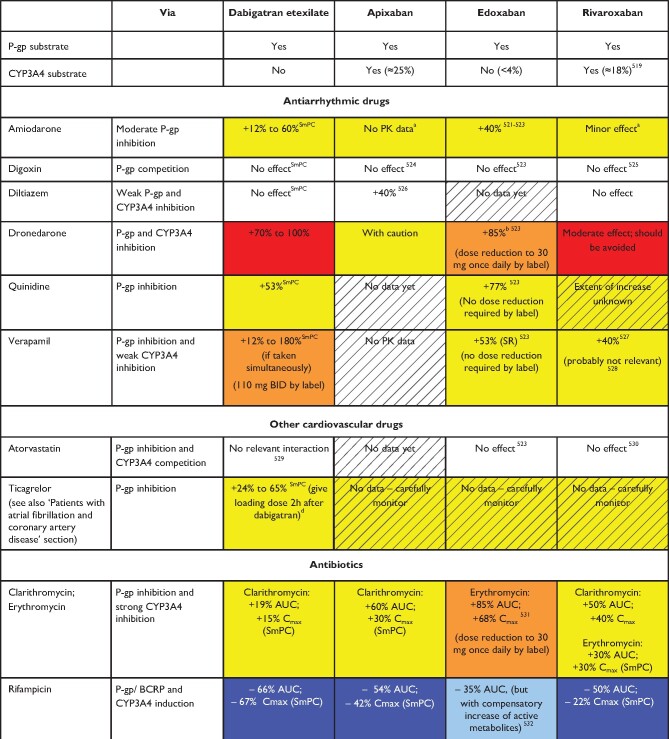
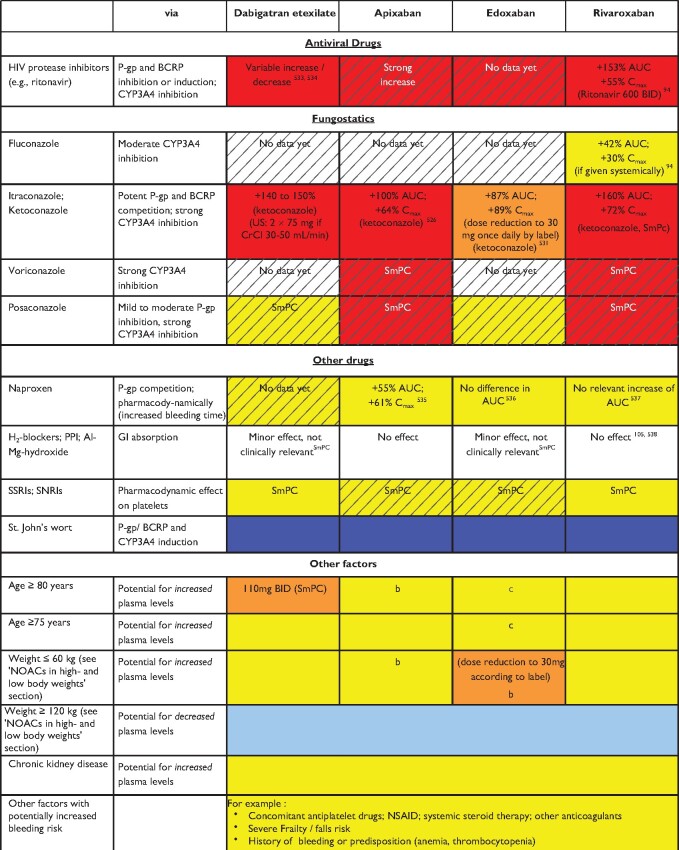
Table 5.
Effect of drug-drug interactions and clinical factors on NOAC plasma levels and anticoagulant effects
|
|
Colour coding is based on the respective NOAC SmPC, drug interaction databases, or expert opinion. The hatched colour coding indicates no clinical or PK data available. Some of the colour codes will likely require adaptation as more data become available over time.
White: No relevant drug–drug interaction anticipated.
Yellow: Caution required, especially in case of polypharmacy or in the presence of ≥2 yellow/bleeding risk factors (see Figure 6).
Orange: Lower dose (dabigatran) or dose reduction (edoxaban) recommended according to label.
Red: Contraindicated/not advisable due to increased plasma levels.
Blue (dark): Contraindicated due to reduced NOAC plasma levels.
Blue (light): Caution required, especially in case of polypharmacy or in the presence of ≥2 light blue interactions due to reduced NOAC plasma levels.
AUC, area under the curve; BCRP, breast cancer resistance protein; BID, twice daily; CrCl, creatinine clearance; NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug; PK, pharmacokinetic; PPI, proton pump inhibitor.
Based on in vitro investigations, comparing the IC50 for P-gp inhibition to maximal plasma levels at therapeutic dose, and/or on interaction analysis of efficacy and safety endpoints in the Phase-3 clinical trials.46,47 No direct PK interaction data available.
Dose reduction based on published criteria (see Table 2).
Age had no significant effect after adjusting for weight and renal function.
Data from Phase I study. Interpret in the light of data from Re-DUAL PCI (see ‘Patients with atrial fibrillation and coronary artery disease' section for details).247
