Abstract
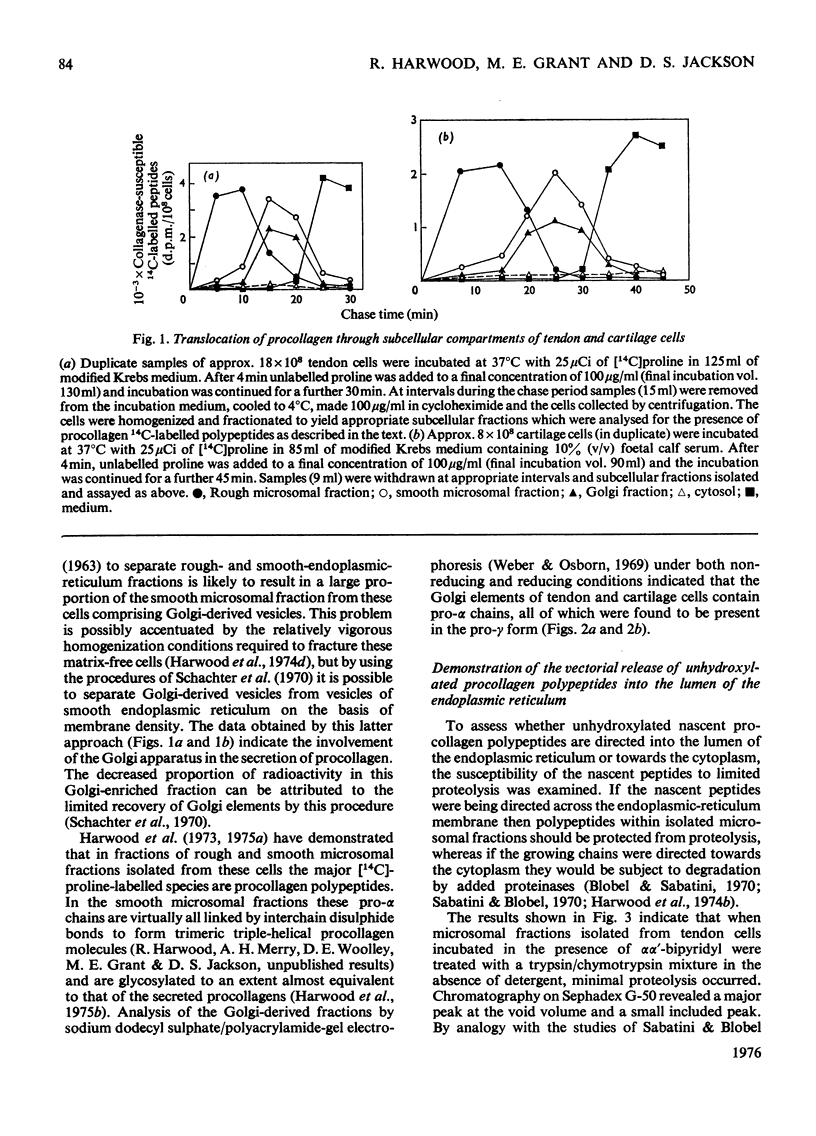
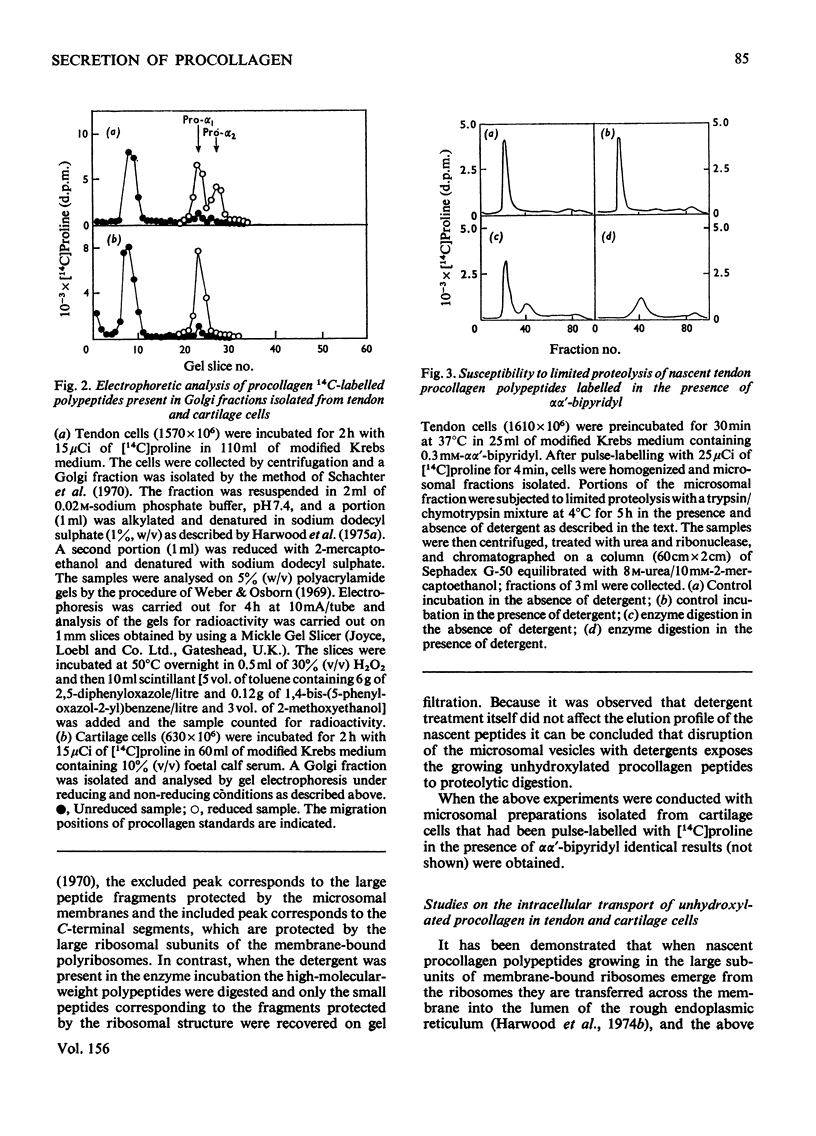
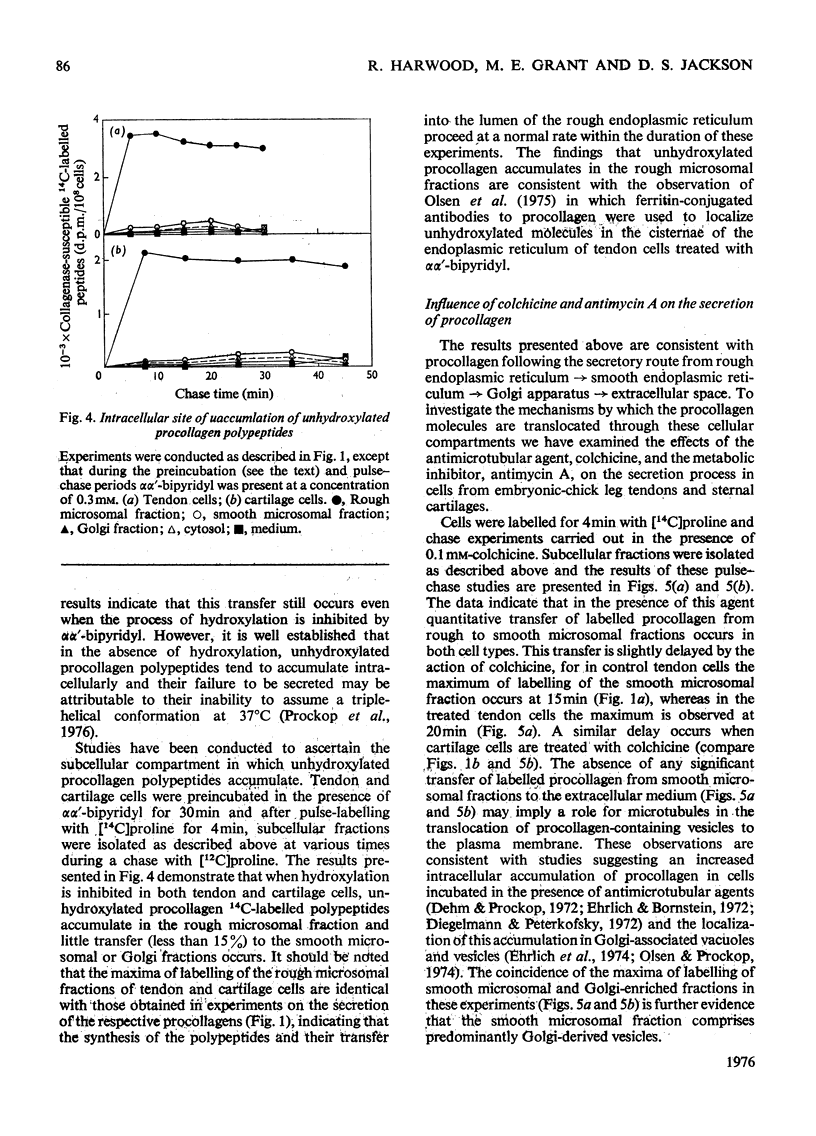
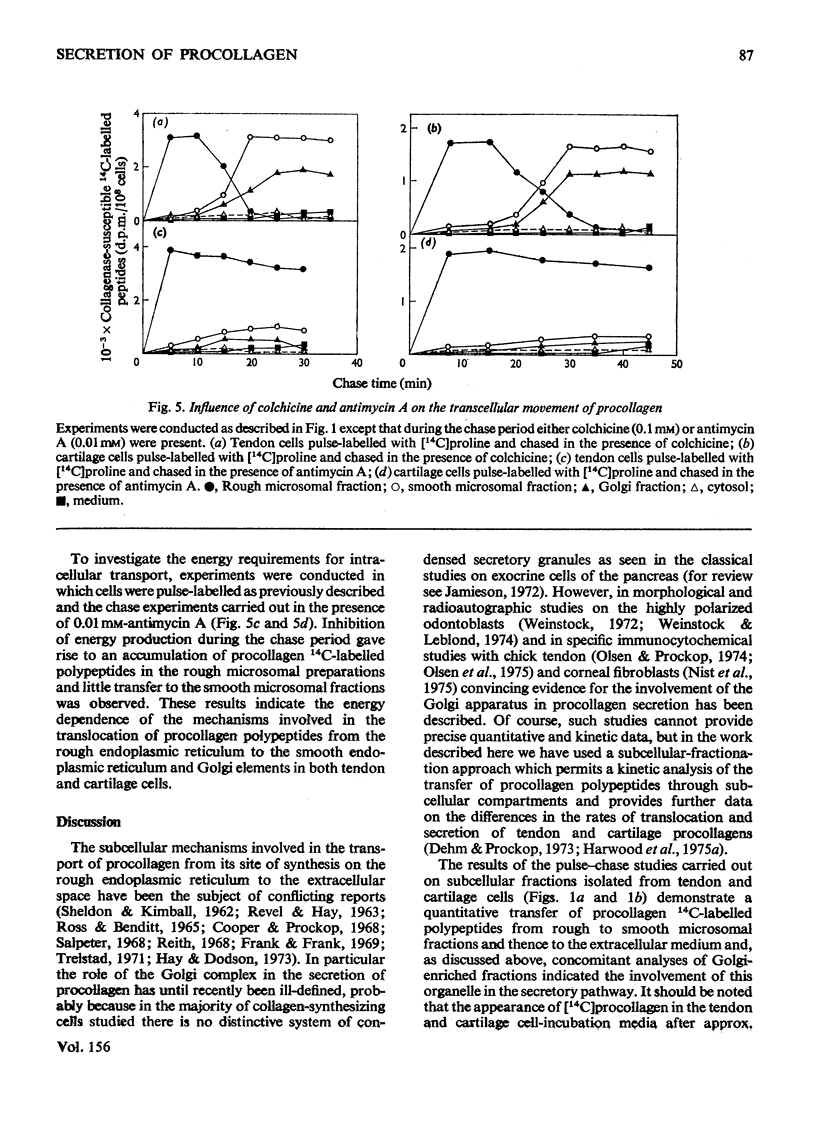
I. Embryonic-chick tendon cells were pulse-labelled for 4 min with [14C]proline and the 14C-labelled polypeptides were chased with unlabelled proline for up to 30 min. Isolation of subcellular fractions during the chase period and their subsequent analysis for bacterial collagenase-susceptible 14C-labelled peptides demonstrated the transfer of procollagen polypeptides from rough to smooth microsomal fractions and thence to the extracellular medium. Parallel analyses of Golgi-enriched fractions indicated the involvement of this organelle in the secretory pathway of procollagen. Sodium dodecylsulphate/polyacrylamide-gel electrophoresis of the 14C-labelled polypeptides present in the Golgi-enriched fractions demonstrated that the procollagen polypeptides were all present as disulphide-linked pro-gamma components. 2. When similar kinetic studies of the intracellular transport of procollagen were conducted with embryonic-chick cartilage cells almost identical results were obtained, but the rate of translocation of cartilage procollagen was significantly slower than that observed for tendon procollagen. 3. When hydroxylation of procollagen polypeptides was inhibited by alphaalpha'-bipyridyl, the nascent polypeptides accumulated in the rough microsomal fraction. 4. When cells were pulse-labelled for 4min with [14C)proline and the label was chased in the presence of colchicine, secretion of procollagen was inhibited and an intracellular accumulation of procollagen 14C-labelled polypeptides was observed in the Golgi-enriched fractions. 5. The energy-dependence of the intracellular transport of procollagen was demonstrated in experiments in which antimycin A was found to inhibit the transfer of procollagen polypeptides from rough to smooth endoplasmic reticulum. 6. It is concluded that procollagen follows the classical route of secretion taken by other extracellular proteins.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. D. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. I. Location of the polypeptides within ribosomes. J Cell Biol. 1970 Apr;45(1):130–145. doi: 10.1083/jcb.45.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. N. Functions of polyribosomes attached to membranes of animal cells. FEBS Lett. 1970 Mar 16;7(1):1–7. doi: 10.1016/0014-5793(70)80603-2. [DOI] [PubMed] [Google Scholar]
- Cooper G. W., Prockop D. J. Intracellular accumulation of protocollagen and extrusion of collagen by embryonic cartilage cells. J Cell Biol. 1968 Sep;38(3):523–537. doi: 10.1083/jcb.38.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALLNER G. STUDIES ON THE STRUCTURAL AND ENZYMIC ORGANIZATION OF THE MEMBRANOUS ELEMENTS OF LIVER MICROSOMES. Acta Pathol Microbiol Scand Suppl. 1963:SUPPL166–SUPPL16694. [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Biosynthesis of cartilage procollagen. Eur J Biochem. 1973 May;35(1):159–166. doi: 10.1111/j.1432-1033.1973.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Bernstein L., Peterkofsky B. Cell-free collagen synthesis on membrane-bound polysomes of chick embryo connective tissue and the localization of prolyl hydroxylase on the polysome-membrane complex. J Biol Chem. 1973 Sep 25;248(18):6514–6521. [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972 Apr;69(4):892–896. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Bornstein P. Microtubules in transcellular movement of procollagen. Nat New Biol. 1972 Aug 30;238(87):257–260. doi: 10.1038/newbio238257a0. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. P., Ross R., Bornstein P. Effects of antimicrotubular agents on the secretion of collagen. A biochemical and morphological study. J Cell Biol. 1974 Aug;62(2):390–405. doi: 10.1083/jcb.62.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. M., Frank P. Autoradiographie quantitative de l'ostéogenèse en microscopie électronique à l'aide de la proline tritiée. Z Zellforsch Mikrosk Anat. 1969;99(1):121–133. [PubMed] [Google Scholar]
- Glaumann H., Ericsson J. L. Evidence for the participation of the Golgi apparatus in the intracellular transport of nascent albumin in the liver cell. J Cell Biol. 1970 Dec;47(3):555–567. doi: 10.1083/jcb.47.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 1. N Engl J Med. 1972 Jan 27;286(4):194–199. doi: 10.1056/NEJM197201272860406. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 2. N Engl J Med. 1972 Feb 3;286(5):242–249. doi: 10.1056/NEJM197202032860505. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 3. N Engl J Med. 1972 Feb 10;286(6):291–300. doi: 10.1056/NEJM197202102860604. [DOI] [PubMed] [Google Scholar]
- HARRIS J. I. Effect of urea on trypsin and alpha-chymotrypsin. Nature. 1956 Mar 10;177(4506):471–473. doi: 10.1038/177471a0. [DOI] [PubMed] [Google Scholar]
- Harwood R., Bhalla A. K., Grant M. E., Jackson D. S. The synthesis and secretion of cartilage procollagen. Biochem J. 1975 Apr;148(1):129–138. doi: 10.1042/bj1480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood R., Connolly A. D., Grant M. E., Jackson D. S. Presumptive mRNA for procollagen: occurrence in membrane bound ribosomes of embryonic chick tendon fibroblasts. FEBS Lett. 1974 Apr 15;41(1):85–88. doi: 10.1016/0014-5793(74)80960-9. [DOI] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. Influence of ascorbic acid on ribosomal patterns and collagen biosynthesis in healing wounds of scorbutic guinea pigs. Biochem J. 1974 Sep;142(3):641–651. doi: 10.1042/bj1420641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. Secretion of procollagen: evidence for the transfer of nascent polypeptides across microsomal membranes of tendon cells. Biochem Biophys Res Commun. 1974 Aug 5;59(3):947–954. doi: 10.1016/s0006-291x(74)80071-9. [DOI] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. Studies on the glycosylation of hydroxylysine residues during collagen biosynthesis and the subcellular localization of collagen galactosyltransferase and collagen glucosyltransferase in tendon and cartilage cells. Biochem J. 1975 Nov;152(2):291–302. doi: 10.1042/bj1520291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The sub-cellular location of inter-chain disulfide bond formation during procollagen biosynthesis by embryonic chick tendon cells. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1188–1196. doi: 10.1016/s0006-291x(73)80020-8. [DOI] [PubMed] [Google Scholar]
- Hay E. D., Dodson J. W. Secretion of collagen by corneal epithelium. I. Morphology of the collagenous products produced by isolated epithelia grown on frozen-killed lens. J Cell Biol. 1973 Apr;57(1):190–213. doi: 10.1083/jcb.57.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. C., Ashton F. E. Studies on acute phase proteins of rat serum. IV. Pathway of secretion of albumin and alpha1-acid glycoprotein from liver. Can J Biochem. 1973 Sep;51(9):1281–1291. doi: 10.1139/o73-168. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S., Harsch M., Rosenbloom J. Hydroxyproline stabilizes the triple helix of chick tendon collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):106–114. doi: 10.1016/0006-291x(73)90960-1. [DOI] [PubMed] [Google Scholar]
- Kruse N. J., Bornstein P. The metabolic requirements for transcellular movement and secretion of collagen. J Biol Chem. 1975 Jul 10;250(13):4841–4847. [PubMed] [Google Scholar]
- Lazarides E., Lukens L. N. Collagen polypeptides: normal release from polysomes in the absence of proline hydroxylation. Science. 1971 Aug 20;173(3998):723–725. doi: 10.1126/science.173.3998.723. [DOI] [PubMed] [Google Scholar]
- Le Marchand Y., Patzelt C., Assimacopoulos-Jeannet F., Loten E. G., Jeanrenaud B. Evidence for a role of the microtubular system in the secretion of newly synthesized albumin and other proteins by the liver. J Clin Invest. 1974 Jun;53(6):1512–1517. doi: 10.1172/JCI107701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Own V., Anderson J. C. The preparation of a bacterial collagenase containing negligible non-specific protease activity. Prep Biochem. 1975;5(3):229–245. doi: 10.1080/00327487508061574. [DOI] [PubMed] [Google Scholar]
- Nist C., Von Der Mark K., Hay E. D., Olsen B. R., Bornstein P., Ross R., Dehm P. Location of procollagen in chick corneal and tendon fibroblasts with ferritin-conjugated antibodies. J Cell Biol. 1975 Apr;65(1):75–87. doi: 10.1083/jcb.65.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Berg R. A., Kishida Y., Prockop D. J. Further characterization of embryonic tendon fibroblasts and the use of immunoferritin techniques to study collagen biosynthesis. J Cell Biol. 1975 Feb;64(2):340–355. doi: 10.1083/jcb.64.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Prockop D. J. Ferritin-conjugated antibodies used for labeling of organelles involved in the cellular synthesis and transport of procollagen. Proc Natl Acad Sci U S A. 1974 May;71(5):2033–2037. doi: 10.1073/pnas.71.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- REVEL J. P., HAY E. D. AN AUTORADIOGRAPHIC AND ELECTRON MICROSCOPIC STUDY OF COLLAGEN SYNTHESIS IN DIFFERENTIATING CARTILAGE. Z Zellforsch Mikrosk Anat. 1963 Oct 8;61:110–144. doi: 10.1007/BF00341524. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Howell K., Palade G. E. Colchicine inhibition of plasma protein release from rat hepatocytes. J Cell Biol. 1975 Jul;66(1):42–59. doi: 10.1083/jcb.66.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith E. J. Collagen formation in developing molar teeth of rats. J Ultrastruct Res. 1967 Dec;21(5):383–414. doi: 10.1016/s0022-5320(67)80148-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Benditt E. P. Wound healing and collagen formation. V. Quantitative electron microscope radioautographic observations of proline-H3 utilization by fibroblasts. J Cell Biol. 1965 Oct;27(1):83–106. doi: 10.1083/jcb.27.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELDON H., KIMBALL F. B. Studies on cartilage. III. The occurrence of collagen within vacuoles of the golgi apparatus. J Cell Biol. 1962 Mar;12:599–613. doi: 10.1083/jcb.12.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Blobel G. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. II. Location of the polypeptides in rough microsomes. J Cell Biol. 1970 Apr;45(1):146–157. doi: 10.1083/jcb.45.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M. H3-proline incorporation into cartilage: electron microscope autoradiographic observations. J Morphol. 1968 Apr;124(4):387–421. doi: 10.1002/jmor.1051240402. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Scherft J. P., Heersche J. N. Accumulation of collagen-containing vacuoles in osteoblasts after administration of colchicine. Cell Tissue Res. 1975;157(3):353–365. doi: 10.1007/BF00225526. [DOI] [PubMed] [Google Scholar]
- Stadler J., Franke W. W. Characterization of the colchicine binding of membrane fractions from rat and mouse liver. J Cell Biol. 1974 Jan;60(1):297–303. doi: 10.1083/jcb.60.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O., Stein Y. Colchicine-induced inhibition of very low density lipoprotein release by rat liver in vivo. Biochim Biophys Acta. 1973 Apr 13;306(1):142–147. doi: 10.1016/0005-2760(73)90219-1. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Intracellular hydroxylation of non-helical protocollagen to form triple-helical procollagen and subsequent secretion of the molecule. Eur J Biochem. 1974 Apr 1;43(2):221–230. doi: 10.1111/j.1432-1033.1974.tb03403.x. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Synthesis and secretion of under-hydroxylated procollagen at various temperatures by cells subject to temporary anoxia. Biochem Biophys Res Commun. 1974 Sep 9;60(1):414–423. doi: 10.1016/0006-291x(74)90220-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weinstock M. Collagen formation--observations on its intracellular packaging and transport. Z Zellforsch Mikrosk Anat. 1972;129(4):455–470. doi: 10.1007/BF00316743. [DOI] [PubMed] [Google Scholar]
- Weinstock M., Leblond C. P. Synthesis, migration, and release of precursor collagen by odontoblasts as visualized by radioautography after (3H)proline administration. J Cell Biol. 1974 Jan;60(1):92–127. doi: 10.1083/jcb.60.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Colchicine-binding protein and the secretion of thyroid hormone. J Cell Biol. 1972 Jul;54(1):157–165. doi: 10.1083/jcb.54.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]
- Wunderlich F., Müller R., Speth V. Direct evidence for a colchicine-induced impairment in the mobility of membrane components. Science. 1973 Dec 14;182(4117):1136–1138. doi: 10.1126/science.182.4117.1136. [DOI] [PubMed] [Google Scholar]


