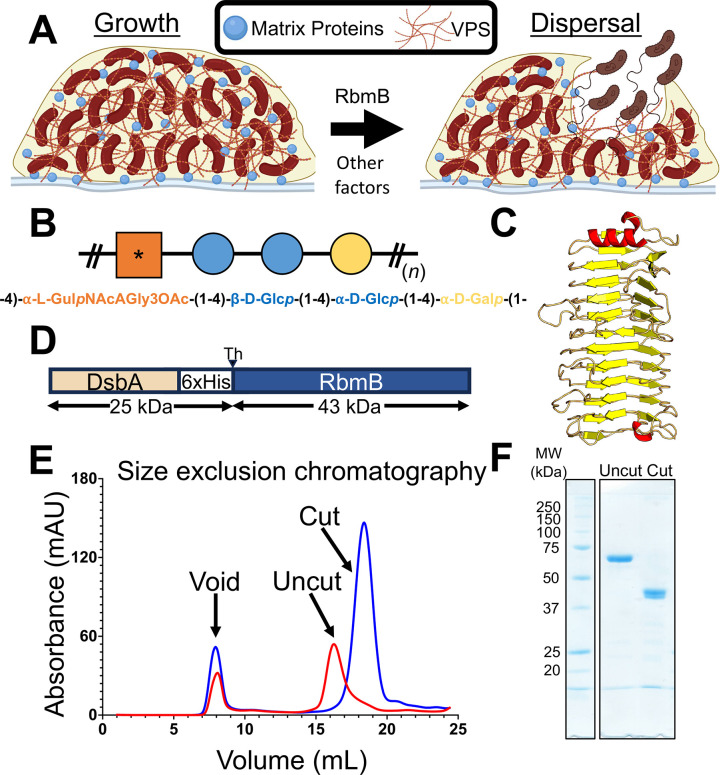
Fig 1. RbmB plays a role in V. cholerae biofilm dispersal.
(A) Schematic of a working model suggesting cellular escape is helped by RbmB-mediated cleavage of VPS. Created in BioRender. Olson, R. (2022) BioRender.com/m42l648 and Olson, R. (2022) BioRender.com/n45c416. (B) Schematic version of the tetrasaccharide structure of VPS based on [21]. The asterisk represents the modified gulose moiety. (C) AlphaFold2 [24] model for RbmB structure. (D) RbmB was expressed as a thrombin-cleavable fusion protein with DsbA and a periplasmic secretion signal peptide at the N-terminus. (E) Size exclusion chromatography trace showing purified RbmB fusion before and after cleavage of the DsbA fusion partner. (F) SDS-PAGE gel showing purified DsbA-RbmB fusion and cleaved and purified RbmB. The expected molecular weight for cleaved RbmB is ~42.7 kDa.