Abstract
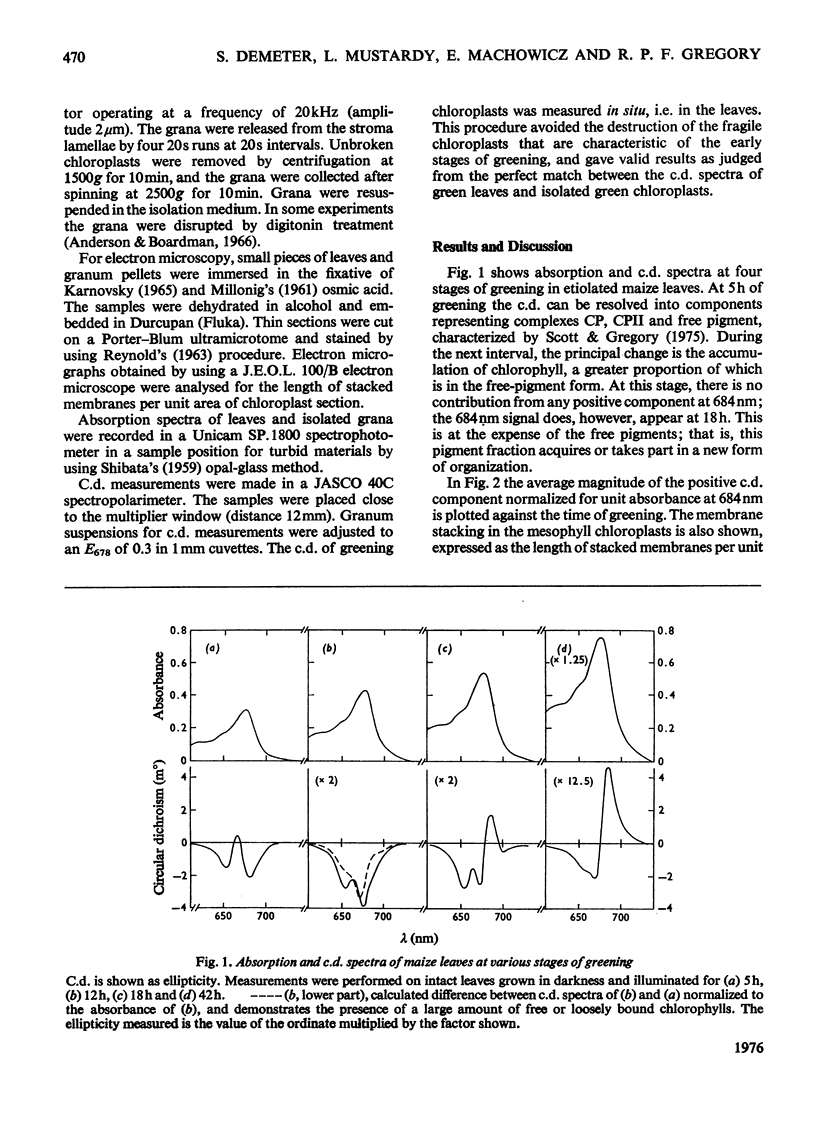
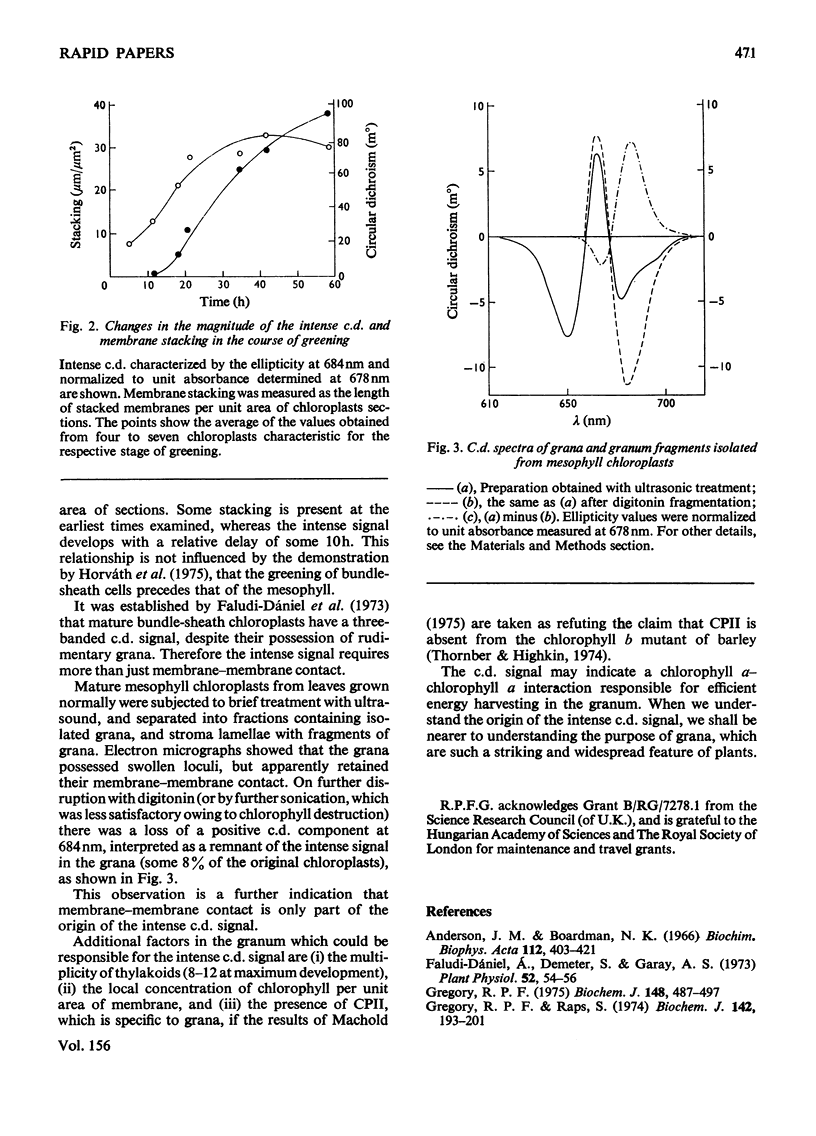
In greening maize mesophyll, circular dichroism (c.d.) revealed the early formation of protein-chlorophyll complexes, followed by unorganized chlorophyll. The intense c.d. [Gregory & Raps (1974) Biochem. J. 142, 193-201] appeared later still, whereas membrane-membrane contact (stacking), measured from electron micrographs, appeared much earlier. Isolated grana, which still showed stacking, lost 92% of their original intense c.d.; intense c.d. is not therefore simply dependent on stacking.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Faludi-Dániel A., Demeter S., Garay A. S. Circular dichroism spectra of granal and agranal chloroplasts of maize. Plant Physiol. 1973 Jul;52(1):54–56. doi: 10.1104/pp.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. P. Evidence that circularly dichroic chlorophyll forms a-682 and a-710 are oriented at right angles to the thylakoid membranes of whole chloroplasts, and that the circular dichroism is light-dependent. Biochem J. 1975 Jun;148(3):487–497. doi: 10.1042/bj1480487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. P., Raps S. The differential scattering of circularly polarized light by chloroplasts and evaluation of their true circular dichroism. Biochem J. 1974 Aug;142(2):193–201. doi: 10.1042/bj1420193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki R., Kunieda R., Takamiya A. Effects of various cations on separation of the two photochemical systems by digitonin treatment. Biochim Biophys Acta. 1971 Jan 12;226(1):144–153. doi: 10.1016/0005-2728(71)90186-1. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B., Gregory R. P. Properties of protein-chlorophyll complexes from pea (Pisum sativum L.) leaves. The organization of chlorophyll. Biochem J. 1975 Aug;149(2):341–347. doi: 10.1042/bj1490341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]


