FIGURE 1.
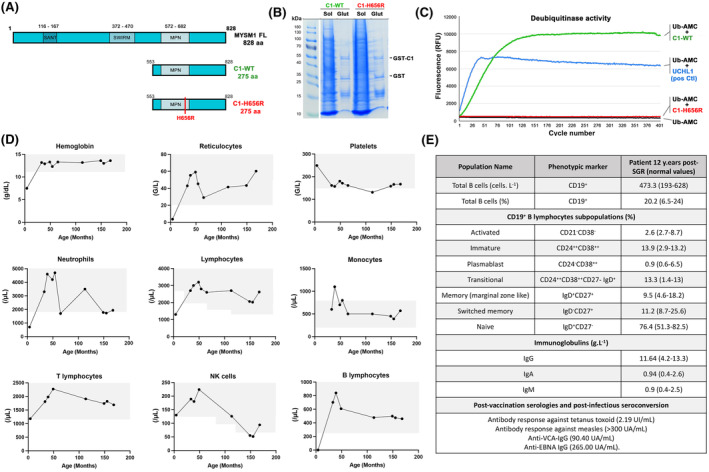
Activity of the H656R MYSM1 variant and haematological parameters in the patient over time. (A) Schematic representation of the full‐length MYSM1 protein as well as the WT and the mutated forms of the C‐terminal part of MYSM1 (noted C1‐WT and C1‐H656R, respectively) used to assess the deubiquitinase activity in vitro. (B) A 6% polyacrylamide gel stained with Coomassie blue showing fractions from a representative purification of GST‐C1‐WT and GST‐C1‐H656R used in in vitro deubiquitinase assay. Glut: Glutathione purification. (C) Fluorescence intensity measurements for C1‐WT (green line), C1‐H656R (red line), UCHL‐1 used as positive control (blue line) and Ub‐AMC alone used as background fluorescence control (black line). (D) Whole blood cell counts over time. Grey areas represent normal values obtained in age‐matched healthy subjects. (E) Immunophenotyping of the B cell compartment in the patient 12 years post‐somatic genetic rescue (SGR) and serology values post‐vaccination and post‐infection. Age‐matched normal values are indicated in brackets.
