Abstract
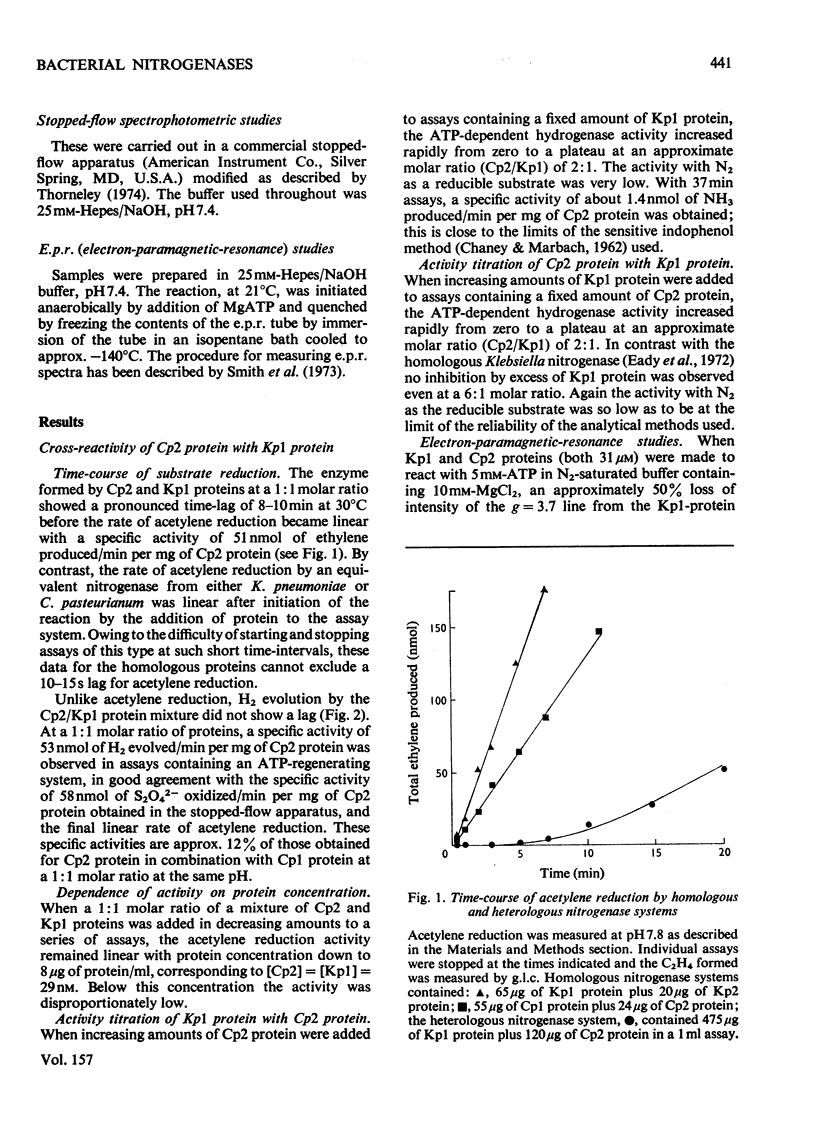
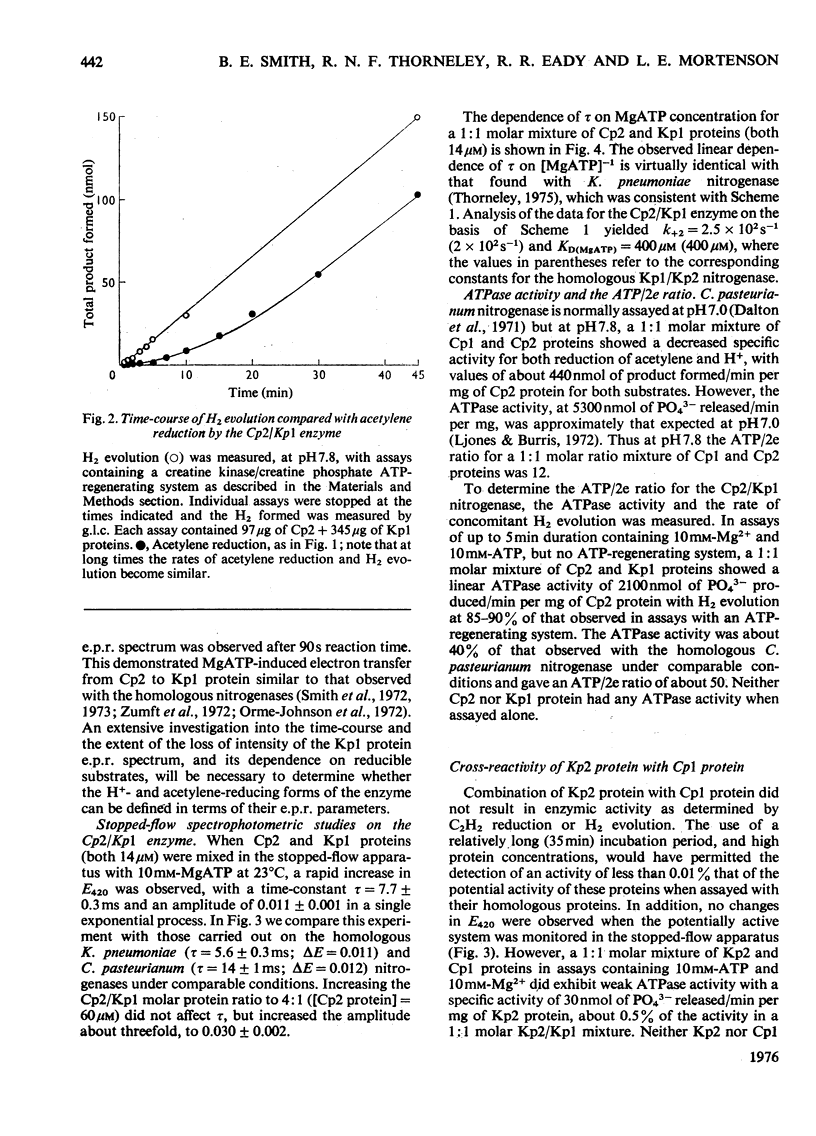
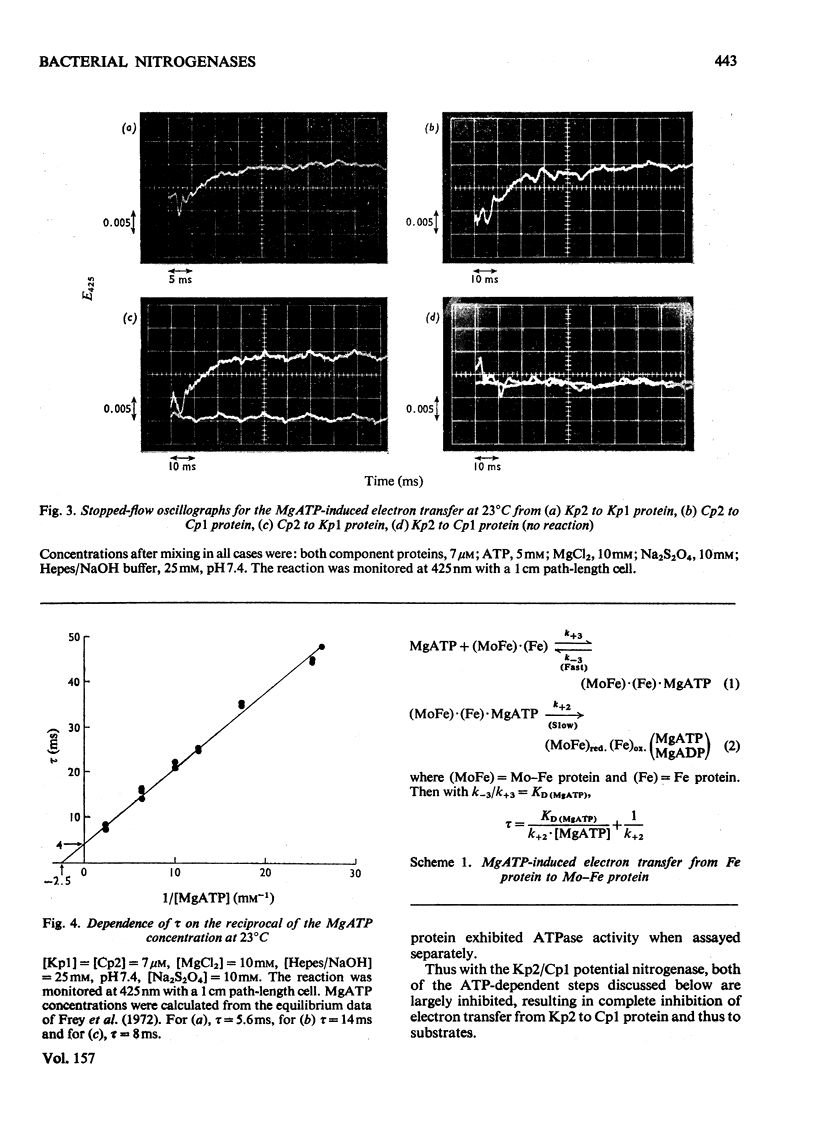
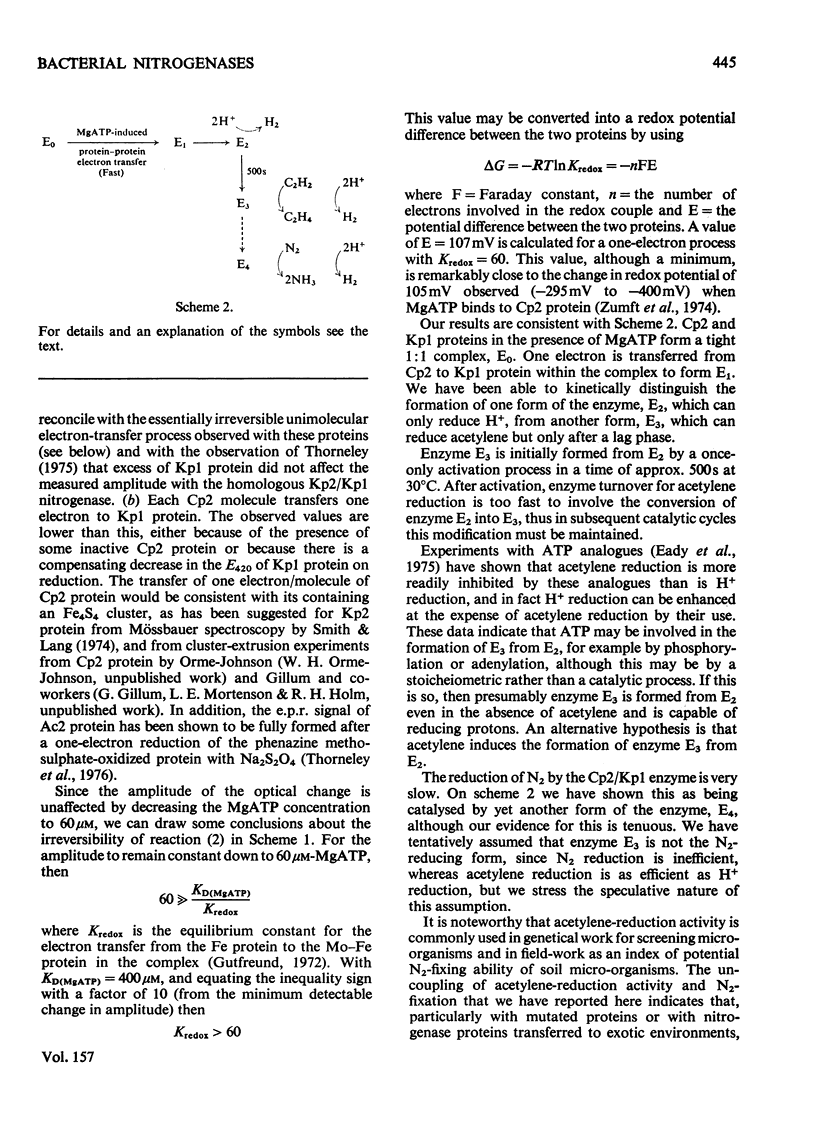
In combination with the Mo-Fe protein of nitrogenase from Klebsiella pneumoniae, the Fe protein of nitrogenase from Clostridium pasteurianum forms an active enzyme with novel properties different from those of either of the homologous nitrogenases. The steady-state rates of reduction of acetylene and H+ are 12% of those of the homologous system from C.pasteurianim. Acetylene reductase activity exhibited an approx. 10min lag at 30 degrees C before the rate of reduction became linear, consistent with a once-only activation step being necessary for acetylene reduction to occur. No such lag was observed for H2 evolution. The activity with N2 as a reducible substrate was very low, implying that acetylene reductase activity is not necessarily an accurate indication of nitrogen-fixing ability. This is of particular relevance to studies on mutant and agronomically important organisms. Stopped-flow spectrophotometric studies showed unimolecular electron transfer from the Fe protein to the Mo-Fe protein to occur at the same rate (k2 = 2.5 X 10(2)s-1) and with the same dependence on ATP concentration (apparent KD = 400 muM) as with the homologous Klebsiella nitrogenase. However, an ATP/2e ratio of 50 was obtained for H2 evolution, indicating that ATP hydrolysis had been uncoupled from electron transfer to substrate. These data indicate that ATP has at least two roles in the mechanism of nitrogenase action. The combination of the Mo-Fe protein of nitrogenase of C.pasteurianim and the Fe protein of K.pneumoniae were inactive in all the above reactions, except for a weak adenosine triphosphatase activity, 0.5% of that of the homologous K.pneumoniae system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggins D. R., Kelly M., Postgate J. R. Resolution of nitrogenase of Mycobacterium flavum 30l into two components and cross reaction with nitrogenase components from other bacteria. Eur J Biochem. 1971 May 11;20(1):140–143. doi: 10.1111/j.1432-1033.1971.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Bui P. T., Mortenson L. E. Mechanism of the enzymic reduction of N2: the binding of adenosine 5'-triphosphate and cyanide to the N2-reducing system. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1021–1027. doi: 10.1073/pnas.61.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Dalton H., Morris J. A., Ward M. A., Mortenson L. E. Purification and some properties of molybdoferredoxin, a component of nitrogenase from Clostridium pasteurianum. Biochemistry. 1971 May 25;10(11):2066–2072. doi: 10.1021/bi00787a016. [DOI] [PubMed] [Google Scholar]
- Detroy R. W., Witz D. F., Parejko R. A., Wilson P. W. Reduction of N2 by complementary functioning of two components from nitrogen-fixing bacteria. Proc Natl Acad Sci U S A. 1968 Oct;61(2):537–541. doi: 10.1073/pnas.61.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Kennedy C., Smith B. E., Thorneley R. N., Yates G., Postgate J. R. Nitrogenase in Azotobacter chroococcum and Klebsiella pneumoniae. Biochem Soc Trans. 1975;3(4):488–492. doi: 10.1042/bst0030488. [DOI] [PubMed] [Google Scholar]
- Eady R. R. Nitrogenase of Klebsiella pneumoniae. Interaction of the component proteins studied by ultracentrifugation. Biochem J. 1973 Nov;135(3):531–535. doi: 10.1042/bj1350531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Postgate J. R. Nitrogenase. Nature. 1974 Jun 28;249(460):805–810. doi: 10.1038/249805a0. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey C. M., Banyasz J. L., Stuehr J. E. Interactions of divalent metal ions with inorganic and nucleoside phosphates. II. Kinetics of magnesium(II) with HP 3 O 10 4- ,ATP, CTP, HP 2 O 7 3- , ADP, and CDP. J Am Chem Soc. 1972 Dec 27;94(26):9198–9204. doi: 10.1021/ja00781a035. [DOI] [PubMed] [Google Scholar]
- Hadfield K. L., Bulen W. A. Adenosine triphosphate requirement of nitrogenase from Azotobacter vinelandii. Biochemistry. 1969 Dec;8(12):5103–5108. doi: 10.1021/bi00840a064. [DOI] [PubMed] [Google Scholar]
- Huang T. C., Zumft W. G., Mortenson L. E. Structure of the molybdoferredoxin complex from Clostridium pasteurianum and isolation of its subunits. J Bacteriol. 1973 Feb;113(2):884–890. doi: 10.1128/jb.113.2.884-890.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. C., Chen C. H., Burris R. H. Inhibition of nitrogenase-catalyzed reductions. Biochim Biophys Acta. 1973 Jan 18;292(1):256–270. doi: 10.1016/0005-2728(73)90270-3. [DOI] [PubMed] [Google Scholar]
- Jeng D. Y., Morris J. A., Mortenson L. E. The effect of reductant in inorganic phosphate release from adenosine 5'-triphosphate by purified nitrogenase of Clostridium pasteurianum. J Biol Chem. 1970 Jun 10;245(11):2809–2813. [PubMed] [Google Scholar]
- Kelly M. Comparisons and cross reactions of nitrogenase from Klebsiella pneumoniae, Azotobacter chroococcum and Bacillus polymyxa. Biochim Biophys Acta. 1969;191(3):527–540. doi: 10.1016/0005-2744(69)90346-5. [DOI] [PubMed] [Google Scholar]
- Ljones T., Burris R. H. ATP hydrolysis and electron transfer in the nitrogenase reaction with different combinations of the iron protein and the molybdenum-iron protein. Biochim Biophys Acta. 1972 Jul 12;275(1):93–101. doi: 10.1016/0005-2728(72)90027-8. [DOI] [PubMed] [Google Scholar]
- Ljones T. Nitrogenase from Clostridium pasteurianum. Changes in optical absorption spectra during electron transfer and effects of ATP, inhibitors and alternative substrates. Biochim Biophys Acta. 1973 Sep 15;321(1):103–113. doi: 10.1016/0005-2744(73)90064-8. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Koch B. L. Compatibility of the components of nitrogenase from soybean bacteroids and free-living nitrogen-fixing bacteria. Biochim Biophys Acta. 1971 Nov 2;253(1):295–297. doi: 10.1016/0005-2728(71)90257-x. [DOI] [PubMed] [Google Scholar]
- Nakos G., Mortenson L. Subunit structure of azoferredoxin from Clostridium pasteurianum W5. Biochemistry. 1971 Feb 2;10(3):455–458. doi: 10.1021/bi00779a016. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Hamilton W. D., Jones T. L., Tso M. Y., Burris R. H., Shah V. K., Brill W. J. Electron paramagnetic resonance of nitrogenase and nitrogenase components from Clostridium pasteurianum W5 and Azotobacter vinelandii OP. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3142–3145. doi: 10.1073/pnas.69.11.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R., Bulen W. A. Kinetic studies of the nitrogense-catalyzed hydrogen volution and nitrogen reduction reactions. Biochemistry. 1970 Sep 15;9(19):3809–3815. doi: 10.1021/bi00821a021. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Lang G. Mössbauer spectroscopy of the nitrogenase proteins from Klebsiella pneumoniae. Structural assignments and mechanistic conclusions. Biochem J. 1974 Feb;137(2):169–180. doi: 10.1042/bj1370169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., Lowe D. J., Bray R. C. Nitrogenase of Klebsiella pneumoniae: electron-paramagnetic-resonance studies on the catalytic mechanism. Biochem J. 1972 Nov;130(2):641–643. doi: 10.1042/bj1300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., Lowe D. J., Bray R. C. Studies by electron paramagnetic resonance on the catalytic mechanism of nitrogenase of Klebsiella pneumoniae. Biochem J. 1973 Oct;135(2):331–341. doi: 10.1042/bj1350331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. V., Telfer A., Evans M. C. Complementary functioning of nitrogenase components from a blue-green alga and a photosynthetic bacterium. J Bacteriol. 1971 Aug;107(2):574–575. doi: 10.1128/jb.107.2.574-575.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Thorneley R. N. A convenient electrochemical preparation of reduced methyl viologen and a kinetic study of the reaction with oxygen using an anaerobic stopped-flow apparatus. Biochim Biophys Acta. 1974 Mar 26;333(3):487–496. doi: 10.1016/0005-2728(74)90133-9. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N., Eady R. R. Nitrogenase of Klebsiella pneumoniae: evidence for an adenosine triphosphate-induced association of the iron-sulphur protein. Biochem J. 1973 Jun;133(2):405–408. doi: 10.1042/bj1330405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Eady R. R., Yates M. G. Nitrogenases of Klebsiella pneumoniae and Azotobacter chroococum. Complex formation between the component proteins. Biochim Biophys Acta. 1975 Oct 22;403(2):269–284. doi: 10.1016/0005-2744(75)90057-1. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N. Nitrogenase of Klebsiella pneumoniae. A stopped-flow study of magnesium-adenosine triphosphate-induce electron transfer between the compeonent proteins. Biochem J. 1975 Feb;145(2):391–396. doi: 10.1042/bj1450391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Willison K. R. Nitrogenase of Klebsiella pneumoniae. Inhibition of acetylene reduction by magnesium ion explained by the formation of an inactive dimagnesium-adenosine triphophate complex. Biochem J. 1974 Apr;139(1):211–214. doi: 10.1042/bj1390211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Yates M. G., Lowe D. J. Nitrogenase of Azotobacter chroococcum. Kinetics of the reduction of oxidized iron-protein by sodium dithionite. Biochem J. 1976 Apr 1;155(1):137–144. doi: 10.1042/bj1550137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso M. Y., Burris R. H. The binding of ATP and ADP by nitrogenase components from Clostridium pasteurianum. Biochim Biophys Acta. 1973 Jun 6;309(2):263–270. doi: 10.1016/0005-2744(73)90024-7. [DOI] [PubMed] [Google Scholar]
- Walker G. A., Mortenson L. E. Effect of magnesium adenosine 5'-triphosphate on the accessibility of the iron of clostridial azoferredoxin, a component of nitrogenase. Biochemistry. 1974 May 21;13(11):2382–2388. doi: 10.1021/bi00708a023. [DOI] [PubMed] [Google Scholar]
- Walker M. N., Mortenson L. E. Evidence for the existence of a fully reduced state of molybdoferredoxin during the functioning of nitrogenase, and the order of electron transfer from reduced ferredoxin. J Biol Chem. 1974 Oct 10;249(19):6356–6358. [PubMed] [Google Scholar]
- Walker M., Mortenson L. E. Oxidation reduction properties of nitrogenase from Clostridium pasteurianum W5. Biochem Biophys Res Commun. 1973 Sep 18;54(2):669–676. doi: 10.1016/0006-291x(73)91475-7. [DOI] [PubMed] [Google Scholar]
- Watt G. D., Bulen W. A., Burns A., Hadfield K. L. Stoichiometry, ATP/2e values, and energy requirements for reactions catalyzed by nitrogenase from Azotobacter vinelandii. Biochemistry. 1975 Sep 23;14(19):4266–4272. doi: 10.1021/bi00690a019. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Cretney W. C., Huang T. C., Mortenson L. E., Palmer G. On the structure and function of nitrogenase from Clostridium pasteurianum W5. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1525–1532. doi: 10.1016/0006-291x(72)90887-x. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Mortenson L. E., Palmer G. Electron-paramagnetic-resonance studies on nitrogenase. Investigation of the oxidation-reduction behaviour of azoferredoxin and molybdoferredoxin with potentiometric and rapid-freeze techniques. Eur J Biochem. 1974 Aug 1;46(3):525–535. doi: 10.1111/j.1432-1033.1974.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Mortenson L. E. The nitrogen-fixing complex of bacteria. Biochim Biophys Acta. 1975 Mar 31;416(1):1–52. doi: 10.1016/0304-4173(75)90012-9. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Mortensson L. E. Evidence for a catalytic-centre heterogeneity of molybdoferredoxin from Clostridium pasteurianum. Eur J Biochem. 1973 Jun 15;35(3):401–409. doi: 10.1111/j.1432-1033.1973.tb02852.x. [DOI] [PubMed] [Google Scholar]



