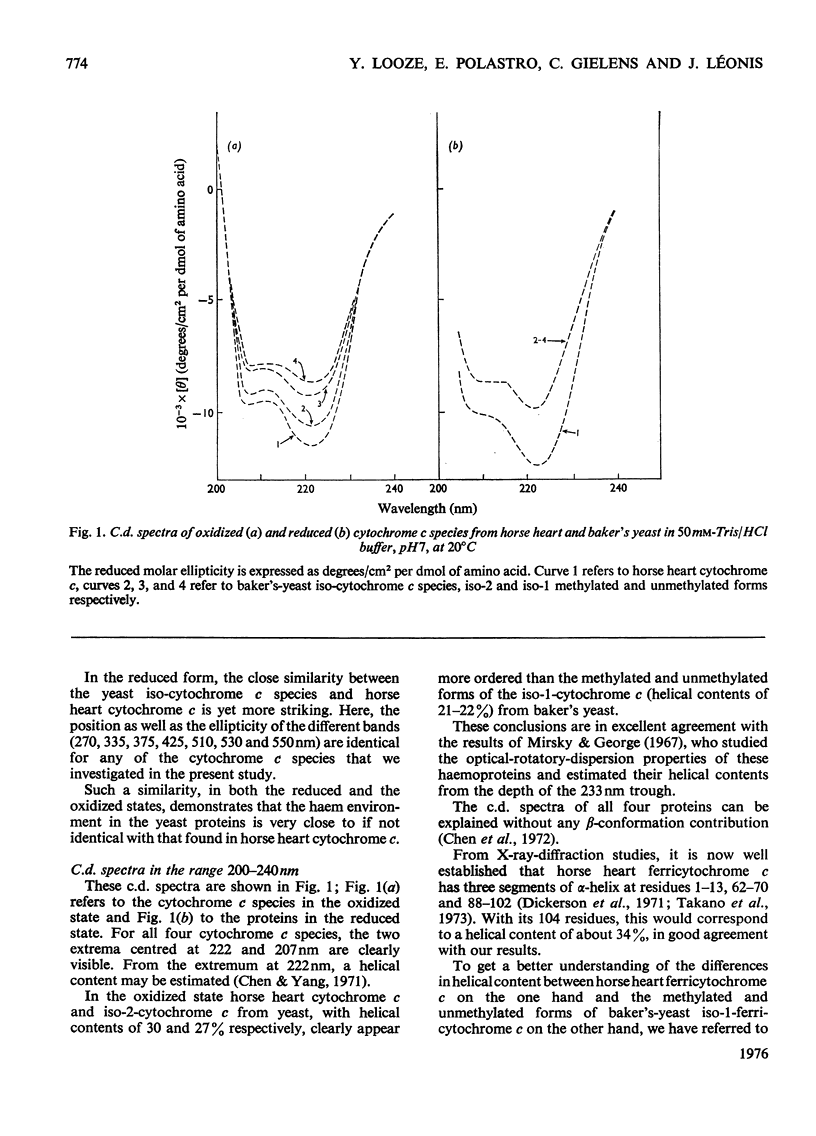
Abstract
The circular-dichroism spectra of baker's-yeast iso-1- (methylated and unmethylated forms) and iso-2-cytochrome c species were examined between 200 and 600nm. In the visible region the yeast haemoproteins have characteristics nearly indistinguishable from those of horse heart cytochrome c. From the spectra in the u.v. region the latter appears, however, to be more helical. It is proposed that the likely element of non-helical structure in iso-1-cytochrome c is residues 62-70.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. H., Yang J. T. A new approach to the calculation of secondary structures of globular proteins by optical rotatory dispersion and circular dichroism. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1285–1291. doi: 10.1016/s0006-291x(71)80225-5. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Glazer A. N., Smith E. L. Identification and location of episilon-N-trimethyllysine in yeast cytochromes c. J Biol Chem. 1970 Jul 10;245(13):3325–3327. [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Foucher M., Verdière J., Lederer F., Slonimski P. P. On the presence of a non-trimethylated iso-1 cytochrome c in a wild-type strain of Saccharomyces cerevisiae. Eur J Biochem. 1972 Nov 21;31(1):139–143. doi: 10.1111/j.1432-1033.1972.tb02511.x. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., SMITH E. L., KREIL G., TUPPY H. Amino-acid sequence of horse heart cytochrome c. Nature. 1961 Dec 23;192:1125–1127. doi: 10.1038/1921125a0. [DOI] [PubMed] [Google Scholar]
- Margalit R., Schejter A. Thermodynamics of the redox reaction of cytochromes c of five different species. FEBS Lett. 1970 Feb 16;6(3):278–280. doi: 10.1016/0014-5793(70)80077-1. [DOI] [PubMed] [Google Scholar]
- Margoliash E., Schejter A. Cytochrome c. Adv Protein Chem. 1966;21:113–286. doi: 10.1016/s0065-3233(08)60128-x. [DOI] [PubMed] [Google Scholar]
- Mirsky R., George P. Optical rotatory dispersion and spectral properties of yeast isocytochromes c. Biochemistry. 1967 Dec;6(12):3671–3675. doi: 10.1021/bi00864a008. [DOI] [PubMed] [Google Scholar]
- Takano T., Kallai O. B., Swanson R., Dickerson R. E. The structure of ferrocytochrome c at 2.45 A resolution. J Biol Chem. 1973 Aug 10;248(15):5234–5255. [PubMed] [Google Scholar]


