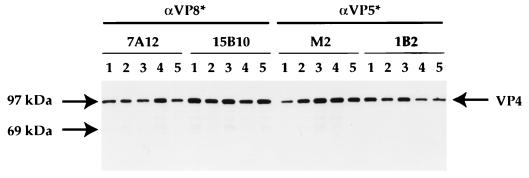
FIG. 2.
Immunoprecipitation of mutant VP4 proteins. The wild-type and mutated VP4 cDNAs were excised as an NcoI/XhoI fragment from pBluescript KS+ (Stratagene, La Jolla, Calif.) and subcloned into a modified pFASTBAC vector (Gibco/BRL). Recombinant baculoviruses were produced in Sf-9 cells (Invitrogen, San Diego, Calif.) with the BAC-to-BAC system (Gibco/BRL) according to the manufacturer’s instructions. To produce recombinant proteins, Sf-9 cells were infected at a multiplicity of infection of 1. At 24 h, the medium was removed and cells were labelled with [35S]methionine (Amersham, Arlington Heights, Ill.), in methionine-free medium (Ex-Cell 401; JRH Biosciences, Lenexa, Kans.) for an additional 48 h. Cells were harvested and lysed, and the proteins were examined by immunoprecipitation (16). The VP4 antibodies, neutralizing MAbs to VP8* (7A12 and 15B10), and MAbs to VP5* (M2 and 1B2) (references 32 and 34 and this paper) were precoupled to Protein A-Sepharose (Sigma Chemical Company) for 2 h at 4°C. The antibody-bead complexes were washed extensively and added to the 35S-labelled lysates, and the samples were immunoprecipitated overnight at 4°C. The samples were washed and then subjected to SDS-PAGE (12% acrylamide; NOVEX, San Diego, Calif.) as described previously (16). The figure shows immunoprecipitation of wild-type VP4 (lane 1), R231H VP4 (lane 2), R241H VP4 (lane 3), R247H VP4 (lane 4), and NC VP4 (lane 5).