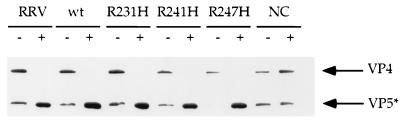
FIG. 3.
Trypsinization of RRV virus and wild-type and mutant VLPs. Wild-type (wt) and mutant VLPs were treated with 10 μg of trypsin per ml (type XIII, N-tosyl-l-phenylalanine chloromethyl ketone [TPCK] treated; Sigma) for 30 min at 37°C (+) or left untreated (−). The reactions were quenched by treatment with an equimolar amount of TLCK (Sigma). Samples were diluted into protein sample buffer, boiled, and subjected to SDS-PAGE as described in the text. The electrophoresed proteins were transferred to nitrocellulose (Schleicher and Schuell, Keene, N.H.), and the VP4 and VP5* proteins were detected with HS-2 (a carboxyl-terminus-specific VP4 monoclonal antibody). The bound antibodies were detected with goat anti-mouse immunoglobulin G coupled to peroxidase (Kirkegaard and Perry, Gaithersburg, Md.). The peroxidase signal was detected by enhanced chemiluminescence (ECL reagent; Pierce Chemical Company, Rockford, Ill.).