Abstract
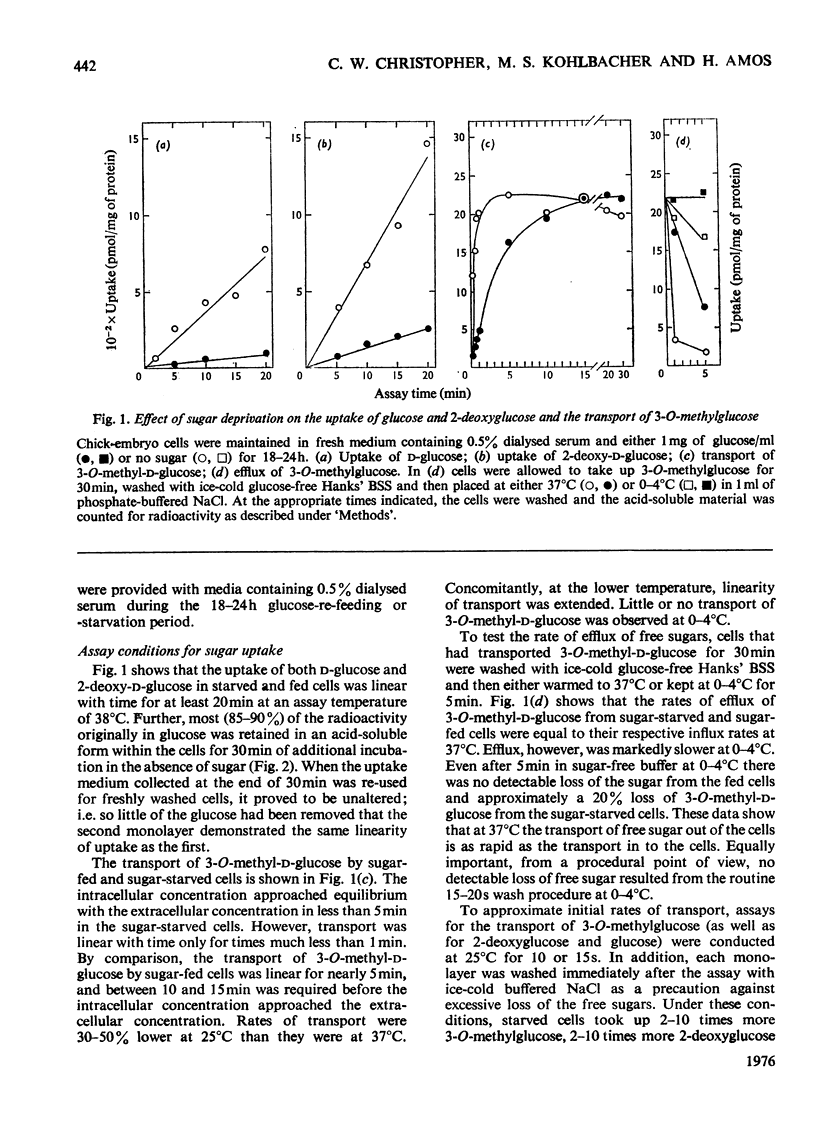
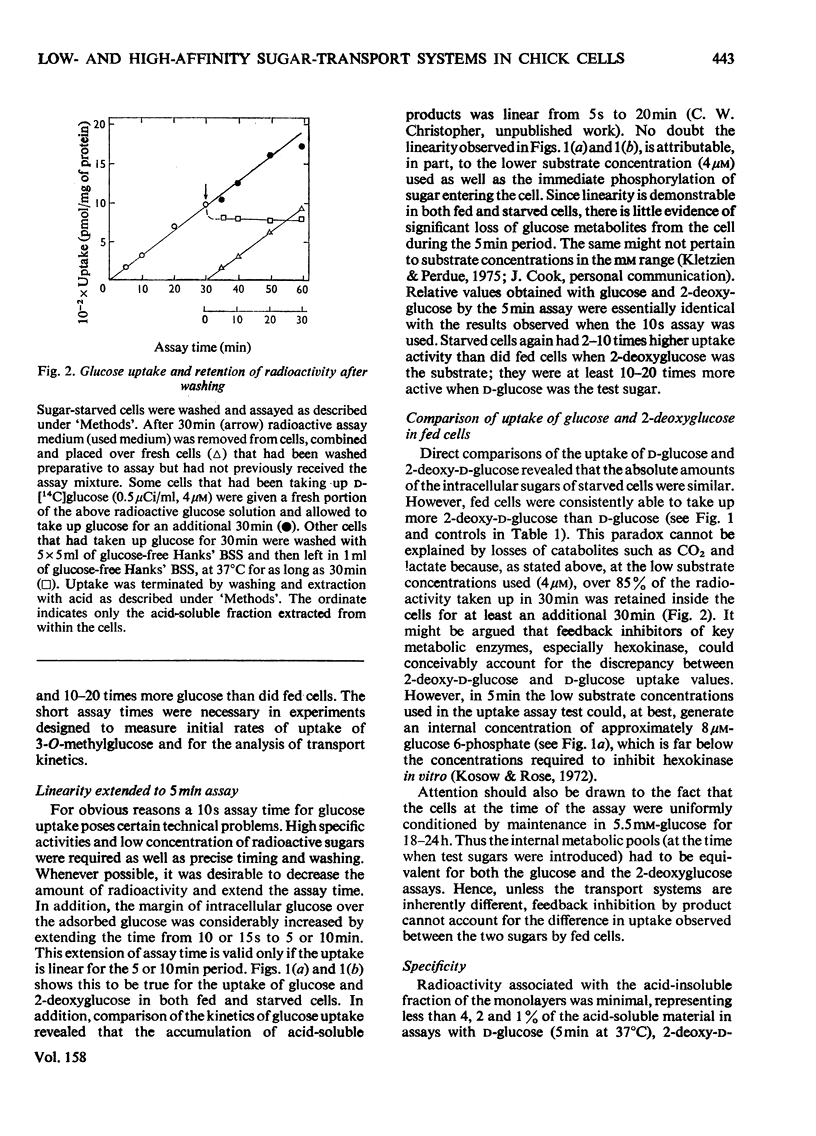
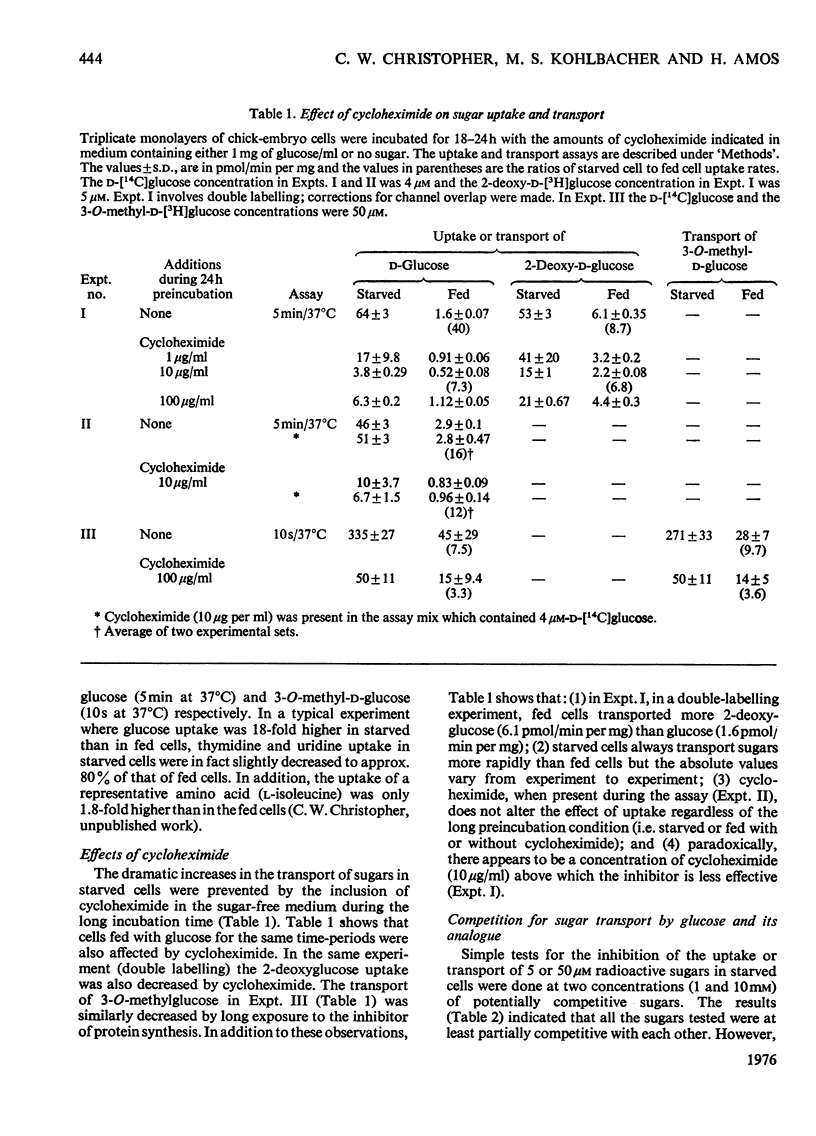
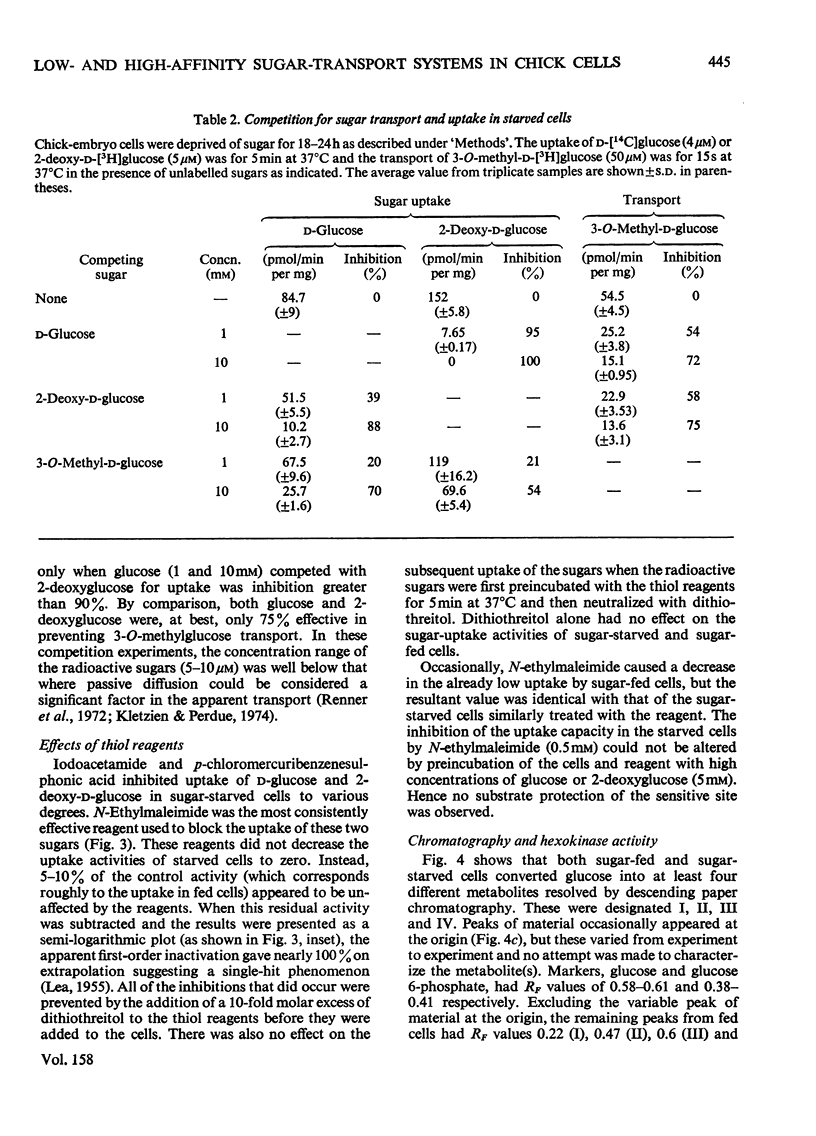
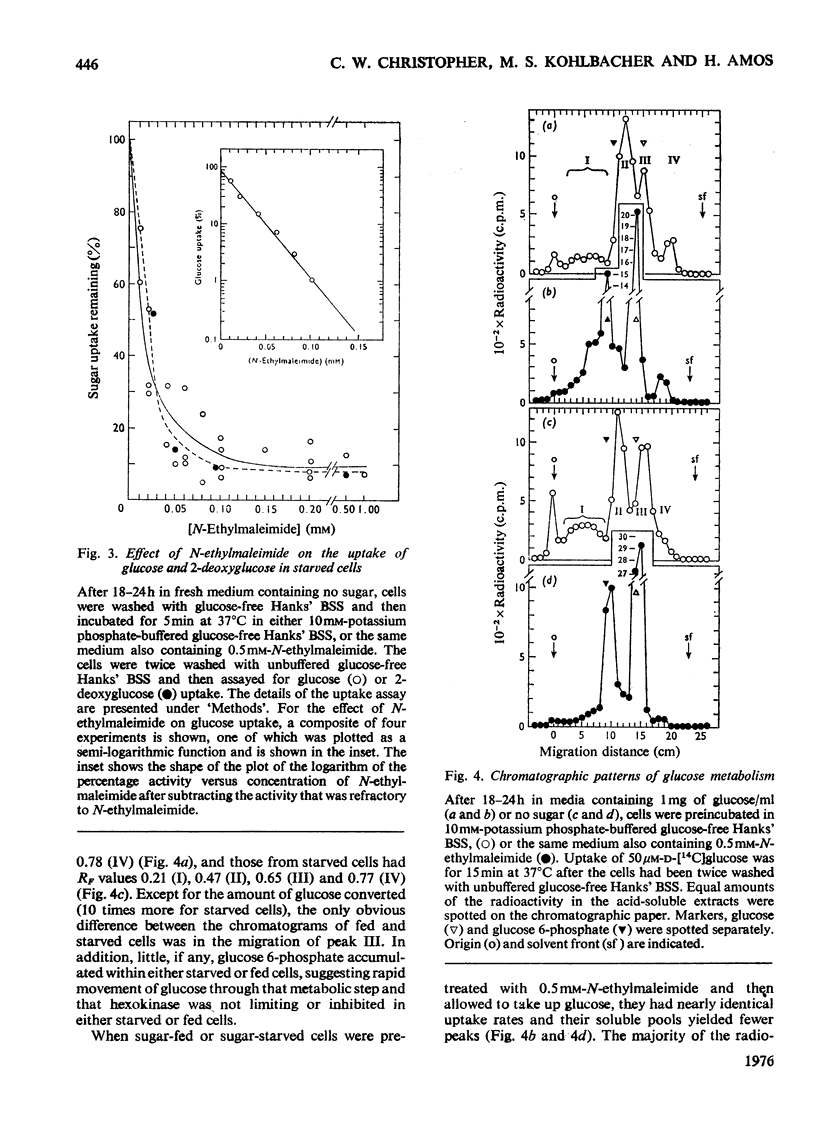
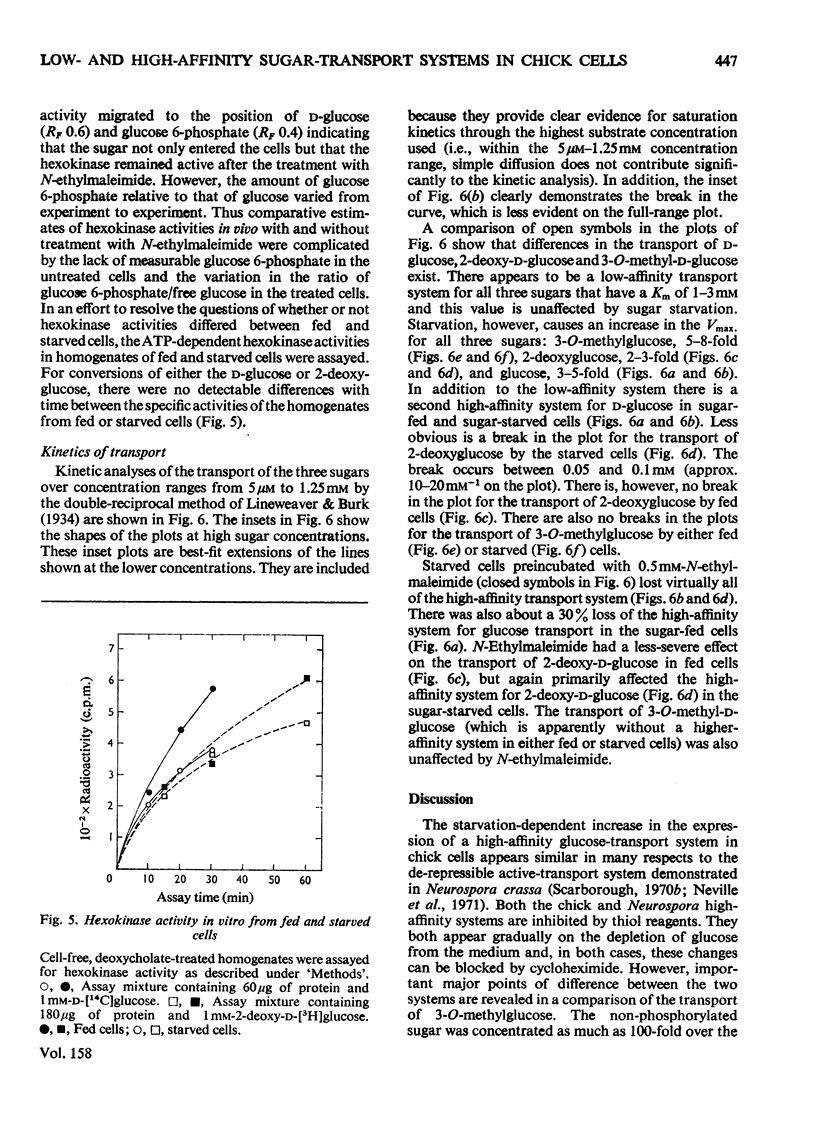
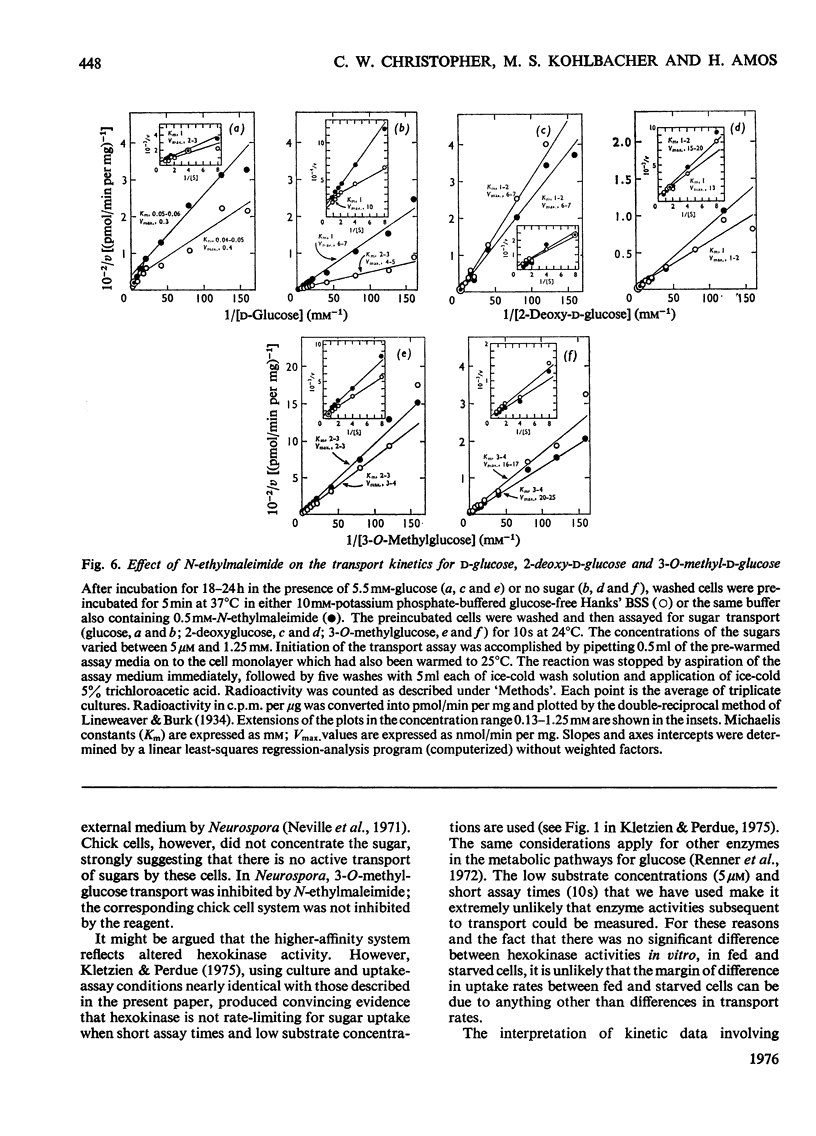
The rate of D-glucose uptake by cells that had been deprived of sugar for 18-24h was consistently observed to be 15-20 times higher than that in control cells maintained for the same length of time in medium containing glucose. This increased rate of glucose transport by sugar-starved cells was due to a 3-5-fold increase in the Vmax. value of a low-affinity system (Km 1 mM) combined with an increase in the Vmax of a separate high-affinity system (Km 0.05-0.2 mM). The high-affinity system, which was most characteristic of starved cells, was particularly sensitive to low concentrations of the thiol reagent N-ethylmaleimide; 50% inhibition of uptake occurred at approx. 0.01 mM-N-ethylmaleimide. In contrast with the high-affinity system, the low-affinity system of either the fed cells or the starved cells was unaffected by N-ethylmaleimide. In addition to the increases in the rate of D-glucose transport, cells deprived of sugar had increased rates of transport of 3-O-methyl-D-glucose and 2-deoxy-D-glucose. No measurable high-affinity transport system could be demonstrated for the transport of 3-O-methylgucose, and N-ethylmaleimide did not alter the initial rate. Thus the transport of 3-O-methyglucose by both fed and starved cells was exclusively by the N-ethylmaleimide-insensitive low-affinity system. The low-affinity system also appeared to be the primary means for the transport of 2-deoxyglucose by fed and starved cells. However, some of the transport of 2-deoxyglucose by starved cells was inhibited by N-ethylmaleimide, suggesting that 2-deoxyglucose may also be transported by a high-affinity system. The results of experiments that measured transport kinetics strongly suggest that glucose can be transported by a least two separate systems, and 3-O-methylglucose and 2-deoxyglucose by one. Support for these interpretations comes from the analysis of the effects of N-ethylmaleimide and cycloheximide as well as from the results of competition experiments. The uptake of glucose is quite different from that of 2-deoxyglucose and 3-O-methylglucose. The net result of sugar starvation serves to emphasize these differences. The apparent de-repression of the transport systems studied presents an interesting basis for further studies of the regulation of transport in a variety of cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMOS H., MOORE M. O. INFLUENCE OF BACTERIAL RIBONUCLEIC ACID ON ANIMAL CELLS IN CULTURE.I. STIMULATION OF PROTEIN SYNTHESIS. Exp Cell Res. 1963 Oct;32:1–13. doi: 10.1016/0014-4827(63)90063-6. [DOI] [PubMed] [Google Scholar]
- Hatanaka M. Sugar effects on murine sarcoma virus transformation. Proc Natl Acad Sci U S A. 1973 May;70(5):1364–1367. doi: 10.1073/pnas.70.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M., Ullrey D., Kijomoto S., Hakomori S. Carbohydrate catabolism and the enhancement of uptake of galactose in hamster cells transformed by polyoma virus. Proc Natl Acad Sci U S A. 1973 Mar;70(3):839–843. doi: 10.1073/pnas.70.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalckar H. M., Ullrey D. Two distinct types of enhancement of galactose uptake into hamster cells: tumor-virus transformation and hexose starvation. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2502–2504. doi: 10.1073/pnas.70.9.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Induction of sugar transport in chick embryo fibroblasts by hexose starvation. Evidence for transcriptional regulation of transport. J Biol Chem. 1975 Jan 25;250(2):593–600. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. I. A functional change in the plasma membrane associated with the rate of cell growth. J Biol Chem. 1974 Jun 10;249(11):3366–3374. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. The inhibition of sugar transport in chick embryo fibroblasts by cytochalasin B. Evidence for a membrane-specific effect. J Biol Chem. 1973 Jan 25;248(2):711–719. [PubMed] [Google Scholar]
- Kosow D. P., Rose I. A. Origin of the delayed feedback control of glucose utilization in ascites tumor cells. Biochem Biophys Res Commun. 1972 Jul 25;48(2):376–383. doi: 10.1016/s0006-291x(72)80061-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martineau R., Kohlbacher M., Shaw S. N., Amos H. Enhancement of hexose entry into chick fibroblasts by starvation: differential effect on galactose and glucose. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3407–3411. doi: 10.1073/pnas.69.11.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B. Differential effect of cytochalasin B on the two possible modes of 2-deoxyglucose transport in HeLa cells. Nat New Biol. 1973 May 23;243(125):125–126. [PubMed] [Google Scholar]
- Neville M. M., Suskind S. R., Roseman S. A derepressible active transport system for glucose in Neurospora crassa. J Biol Chem. 1971 Mar 10;246(5):1294–1301. [PubMed] [Google Scholar]
- PALADINI A. C., LELOIR L. F. Studies on uridine-diphosphate-glucose. Biochem J. 1952 Jun;51(3):426–430. doi: 10.1042/bj0510426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Scarborough G. A. Sugar transport in Neurospora crassa. II. A second glucose transport system. J Biol Chem. 1970 Aug 10;245(15):3985–3987. [PubMed] [Google Scholar]
- Scarborough G. A. Sugar transport in Neurospora crassa. J Biol Chem. 1970 Apr 10;245(7):1694–1698. [PubMed] [Google Scholar]
- Shaw S. N., Amos H. Insulin stimulation of glucose entry in chick fibroblasts and HeLa cells. Biochem Biophys Res Commun. 1973 Jul 2;53(1):357–365. doi: 10.1016/0006-291x(73)91441-1. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Rucoslahti E., Nordling S. Neuraminidase stimulates division and sugar uptake in density-inhibited cell cultures. Nat New Biol. 1972 Aug 16;238(85):211–212. doi: 10.1038/newbio238211a0. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]


