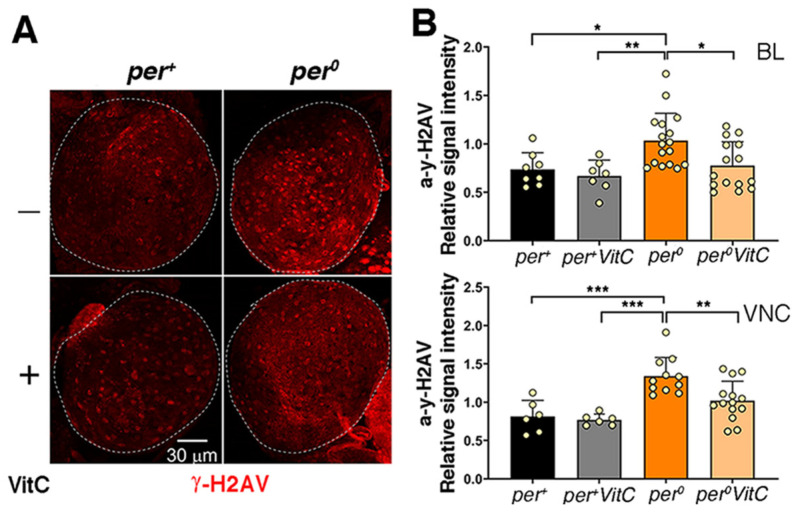
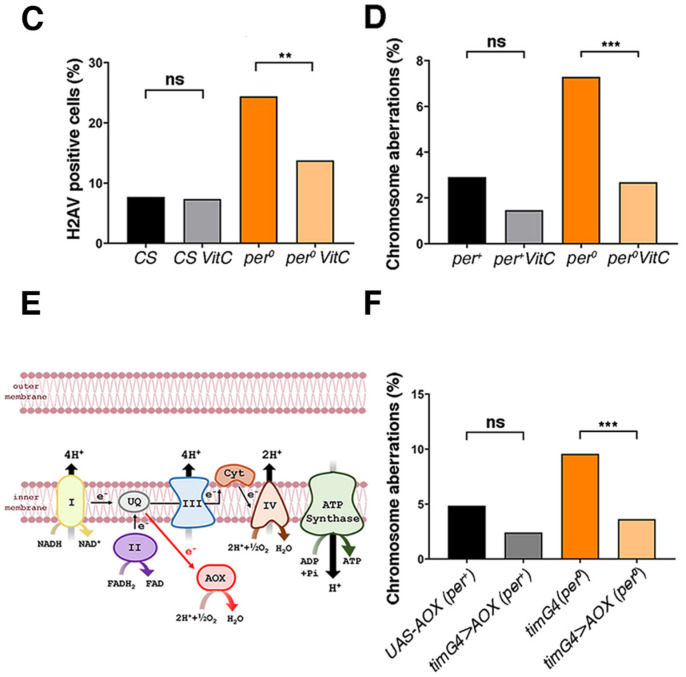
Figure 3.
ROS buffering and reduction decrease DNA damage and chromosome aberrations in per0. Treatment with vitamin C (VitC, 40 mM in standard medium, from embryo) reduced anti-γ-H2AV immune labelling (A–C) and chromosome aberrations (D) in per0 third-instar larva males. (A) Brain lobes (BLs) from third-instar per+ and per0 male larvae obtained by reciprocal crossing (♀ CS × ♂ per0 and vice versa); anti-γ-H2AV on the whole mount. Confocal maximum intensity projections. Dashed lines outline the BLs. Size bar = 30 μm. ZT = 2. (B) Quantification of anti-γ-H2AV immune fluorescence intensity [relative signal intensity = (signal-background)/background] in whole-mount CNS from third-instar per+ and per0 male larvae as above. Normal distribution of data was confirmed with the Shapiro–Wilk test. Top, BL (one per individual). Two-way ANOVA, Genotype (F1, 43 = 7.318, p = 0.0097), VitC treatment (F1, 43 = 4.732, p = 0.0352), Genotype × VitC treatment (F1, 43 = 1.617, p = 0.2104). Tukey’s multiple comparisons test, per+ vs. per0, * p = 0.0282; per+ VitC vs. per0, ** p = 0.0075; per0 vs. per0 VitC, * p = 0.0196. Bottom, VNC. Two-way ANOVA, Genotype (F1, 33 = 23.75, p < 0.0001), VitC treatment (F1, 33 = 5.241, p = 0.0286), Genotype × VitC treatment (F1, 33 = 3.044, p = 0.0903). Tukey’s multiple comparisons test, per+ vs. per0, *** p = 0.0003; per+ VitC vs. per0, *** p = 0.0001; per0 vs. per0 VitC, ** p = 0.0066. ZT = 1. (C) Proportion of anti-γ-H2AV immune positive cells in CNS squash preparations from third-instar male larvae. Cells were considered ‘H2AV positive’ when the relative intensity of the anti-γ-H2AV immune signal in the nucleus [(signal-background)/background] was equal or more than 1.5. The DAPI signal was used to identify nuclei. Treatment with VitC did not affect CS (Fisher’s exact test, p = 0.9209) but lowered the proportion of anti-γ-H2AV immune labelled cells in per0 (Fisher’s exact test, ** p = 0.0011). Total number of cells scored (from left to right), n = 912, 775, 295, 298. ZT = 2. (D) Proportion of chromosome aberrations [(abnormal metaphases/total metaphases) × 100] in CNS squash preparations from third-instar per+ and per0 male larvae obtained by reciprocal crossing (♀ CS × ♂ per0 and vice versa). Treatment with VitC lowered the proportion of chromosome aberrations. The effect was small in per+ (Fisher’s exact test, p = 0.1886) but highly significant in per0 (Fisher’s exact test, *** p < 0.0005). Total number of metaphases scored (from left to right), n = 468, 440, 439, 633. ZT = 1. AOX overexpression rescues chromosome aberrations (E,F). (E) Cartoon showing the position of the Alternative Oxidase (AOX) in the electron transport chain. (F) The overexpression of AOX using the pan-circadian tim-GAL4 (timG4 > AOX) driver reduced the frequency of aberrations. The effect was marginal in per+ (Fisher’s exact test, p = 0.0663) but highly significant in per0 (Fisher’s exact test, *** p < 0.0009). Total number of metaphases scored (from left to right), n = 397, 387, 456, 412. ZT = 1. Males were obtained by reciprocal crossing [♀ UAS-AOX (per+) × ♂ tim-GAL4 (per0) and vice versa].