Abstract
1. The primary structure of a 4Fe-4S ferredoxin from Bacillus stearothermophilus was determined and shown to consist of a single polypeptide chain of 81 amino acid residues. The molecular weight of the holoprotein is about 9120. 2. There are only four cysteine residues in the molecule; three of these are located near the N-terminus as a Cys-X-X-Cys-X-X-Cys segment, and the fourth cysteine residue is followed by a proline and located in the C-terminal half. 3. The Fe-S chromophore in B. stearothermophilus ferredoxin was previously well characterized and was shown to consist of a single 4Fe-4S cluster. This ferredoxin sequence establishes for the first time the relative location of the four cysteine residues necessary to bind the 4Fe-4S cluster of a 4Fe ferredoxin, and is in agreement with the criteria for the relative positions of the cysteines proposed from X-ray-crystallographic studies on an 8Fe (two 4Fe-4S clusters) ferredoxin. 4. The sequence of B. stearothermophilus ferredoxin is homologous in many segments to that of other bacterial ferredoxins, the degree of homology being greater towards ferredoxins from Desulfovibrio gigas and photosynthetic bacteria than to Clostridial ferredoxins. 5. The presence of a relatively higher number of glutamic acid and lower number of cysteine residues in the molecule may explain the greater thermal stability and oxygen-insenstivity of this ferredoxin.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Sieker L. C., Jensen L. H. Structure of a bacterial ferredoxin. J Biol Chem. 1973 Jun 10;248(11):3987–3996. [PubMed] [Google Scholar]
- Blombäck B., Blombäck M., Edman P., Hessel B. Human fibrinopeptides. Isolation, characterization and structure. Biochim Biophys Acta. 1966 Feb 28;115(2):371–396. doi: 10.1016/0304-4165(66)90437-5. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Kolb E., Harris J. I. Amino acid sequence homology in alcohol dehydrogenase. FEBS Lett. 1973 Jun 15;33(1):1–3. doi: 10.1016/0014-5793(73)80144-9. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Cammack R., Hall D. O., Mullinger R. N., Rao K. K. Ferredoxin from Bacillus stearothermophilus. Experientia Suppl. 1976;26:307–313. doi: 10.1007/978-3-0348-7675-9_25. [DOI] [PubMed] [Google Scholar]
- Devenathan T., Akagi J. M., Hersh R. T., Himes R. H. Ferredoxin from two thermophilic clostridia. J Biol Chem. 1969 Jun 10;244(11):2846–2853. [PubMed] [Google Scholar]
- Hall D. O., Rao K. K., Cammack R. The iron-sulphur proteins: structure, function and evolution of a ubiquitous group of proteins. Sci Prog. 1975 Summer;62(246):285–317. [PubMed] [Google Scholar]
- Hase T., Wada K., Matsubara H. Amino acid sequence of the major component of Aphanothece sacrum ferredoxin. J Biochem. 1976 Feb;79(2):329–343. doi: 10.1093/oxfordjournals.jbchem.a131076. [DOI] [PubMed] [Google Scholar]
- Hocking J. D., Harris J. I. Glyceraldehyde 3-phosphate dehydrogenase from an extreme thermophile, Thermus aquaticus. Experientia Suppl. 1976;26:121–133. doi: 10.1007/978-3-0348-7675-9_10. [DOI] [PubMed] [Google Scholar]
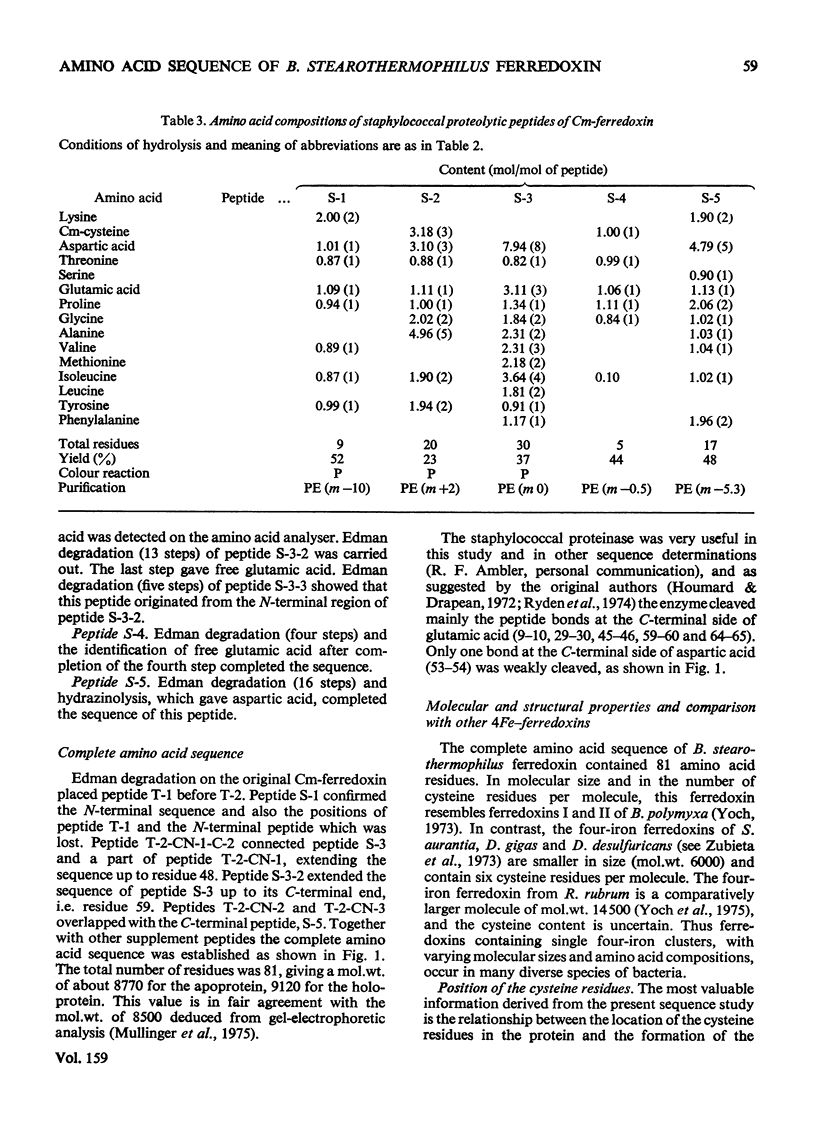
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. W., Canale-Parola E. Properties of rubredoxin and ferredoxin isolated from spirochetes. Arch Mikrobiol. 1973;89(4):341–353. doi: 10.1007/BF00408901. [DOI] [PubMed] [Google Scholar]
- Koide T., Tsunasawa S., Ikenaka T. Studies on soybean trypsin inhibitors. 2. Amino-acid sequence around the reactive site of soybean trypsin inhibitor (Kunitz). Eur J Biochem. 1973 Feb 1;32(3):408–416. doi: 10.1111/j.1432-1033.1973.tb02623.x. [DOI] [PubMed] [Google Scholar]
- Le Gall J., Dragoni N. Dependance of sulfite reduction on a crystallized ferredoxin from Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Apr 19;23(2):145–149. doi: 10.1016/0006-291x(66)90519-5. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M., Chain R. K. Spinach ferredoxin. I. Amino acid composition and terminal sequences. J Biol Chem. 1968 Apr 25;243(8):1725–1731. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. Spinach ferredoxin. II. Typtic, chymotryptic, and thermolytic peptides, and complete amino acid sequence. J Biol Chem. 1968 Apr 25;243(8):1732–1757. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M., Tsuchiya D. K., Evans M. C. The amino acid sequence of Chromatium ferredoxin. J Biol Chem. 1970 Apr 25;245(8):2121–2131. [PubMed] [Google Scholar]
- Mullinger R. N., Cammack R., Rao K. K., Hall D. O., Dickson D. P., Johnson C. E., Rush J. D., Simopoulos A. Physicochemical characterization of the four-iron-four-sulphide ferredoxin from Bacillus stearothermophilus. Biochem J. 1975 Oct;151(1):75–83. doi: 10.1042/bj1510075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Raidt H. Stereochemical basis of heat stability in bacterial ferredoxins and in haemoglobin A2. Nature. 1975 May 15;255(5505):256–259. doi: 10.1038/255256a0. [DOI] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in proteins: possible implications for the mechanism of formation of helical regions. J Mol Biol. 1971 May 28;58(1):237–259. doi: 10.1016/0022-2836(71)90243-9. [DOI] [PubMed] [Google Scholar]
- Rydén A. C., Rydén L., Philipson L. Isolation and properties of a staphylococcal protease, preferentially cleaving glutamoyl-peptide bonds. Eur J Biochem. 1974 May 2;44(1):105–114. doi: 10.1111/j.1432-1033.1974.tb03462.x. [DOI] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T., Evans M. C., Rao K. K. Amino acid sequence of ferredoxin from a photosynthetic green bacterium, Chlorobium limicola. Biochemistry. 1974 Jul 2;13(14):2953–2959. doi: 10.1021/bi00711a026. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T., Evans M. C., Rao K. K. The amino acid sequence of ferredoxin II from Chlorobium limicola, a photosynthetic green bacterium. Biochemistry. 1975 May 6;14(9):1938–1943. doi: 10.1021/bi00680a021. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T., Himes R. H., Akagi J. M. The primary structure of the Clostridium thermosaccharolyticum ferredoxin, a heat-stable ferredoxin. J Biol Chem. 1973 Aug 10;248(15):5215–5217. [PubMed] [Google Scholar]
- Travis J., Newman D. J., LeGall J., Peck H. D., Jr The amino acid sequence of ferredoxin from the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1971 Oct 15;45(2):452–458. doi: 10.1016/0006-291x(71)90840-0. [DOI] [PubMed] [Google Scholar]
- Tsugita A., Gish D. T., Young J., Fraenkel-Conrat H., Knight C. A., Stanley W. M. THE COMPLETE AMINO ACID SEQUENCE OF THE PROTEIN OF TOBACCO MOSAIC VIRUS. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1463–1469. doi: 10.1073/pnas.46.11.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooten M., De Coen J. L. Etude conformationnelle d'une ferredoxine bactérielle. Arch Int Physiol Biochim. 1974 Oct;82(4):793–793. [PubMed] [Google Scholar]
- Vandekerckhove J., Van Montagu M. Sequence analysis of fluorescamine-stained peptides and proteins purified on a nanomole scale. Application to proteins of bacteriophage MS2. Eur J Biochem. 1974 May 2;44(1):279–288. doi: 10.1111/j.1432-1033.1974.tb03483.x. [DOI] [PubMed] [Google Scholar]
- Wada K., Kagamiyama H., Shin M., Matsubara H. Ferredoxin from a blue-green alga, Aphanothece sacrum (Suringar) Okada. J Biochem. 1974 Dec;76(6):1217–1225. doi: 10.1093/oxfordjournals.jbchem.a130674. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I., Sweeney W. V. Characterization of two soluble ferredoxins as distinct from bound iron-sulfur proteins in the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1975 Nov 10;250(21):8330–8336. [PubMed] [Google Scholar]
- Yoch D. C. Purification and properties of two ferredoxins from the nitrogen-fixing bacterium Bacillus polymyxa. Arch Biochem Biophys. 1973 Oct;158(2):633–640. doi: 10.1016/0003-9861(73)90555-9. [DOI] [PubMed] [Google Scholar]
- Zubieta J. A., Mason R., Postgate J. R. A four-iron ferredoxin from Desulfovibrio desulfuricans. Biochem J. 1973 Aug;133(4):851–854. doi: 10.1042/bj1330851. [DOI] [PMC free article] [PubMed] [Google Scholar]


