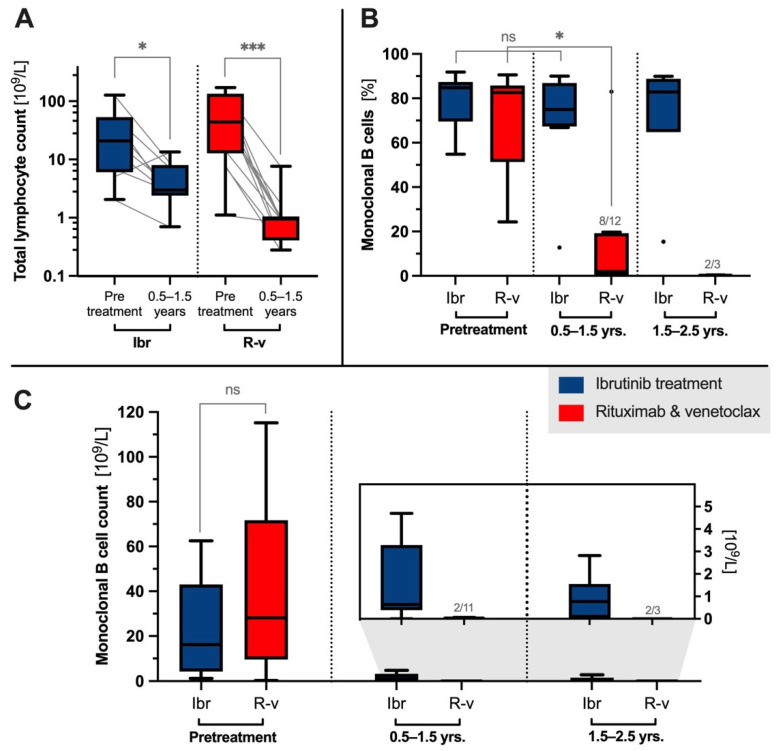
Figure 1.
Clonal kinetics following administration of ibrutinib (Ibr) or rituximab–venetoclax (R–v). Total lymphocyte counts before treatment in ibrutinib-treated versus R–v (pranksum = 0.21), with significant (signed-rank) median reductions in lymphocyte counts from 20.90 × 10⁹/L (n = 9) to 2.98 × 10⁹/L (n = 9) for ibrutinib and from 43.99 × 10⁹/L (n = 14) to 0.95 × 10⁹/L (n = 12) for rituximab–venetoclax (R–v) at follow-up of 0.5 to 1.5 years (A). The monoclonal burden was 75% at first follow-up in the ibrutinib group (n = 9) compared with 1.99% in the R–v group (n = 12), with residual disease undetectable in 4 of 12 patients in the R–v group at 0.5 to 1.5 years (B). Estimated monoclonal CLL cell counts from flow cytometry: 16.2 × 10⁹/L (n = 9) for ibrutinib and 28.2 × 10⁹/L (n = 15) for R–v before treatment, decreasing to 0.6 × 10⁹/L for ibrutinib (n = 9) and near the detection limit for R–v (n = 11) during follow-up of 0.5 to 1.5 years (C). Abbreviations *: p < 0.05, ***: p < 0.005, ns: not significant. Dotted lines denote separate treatment groups or follow-up intervals.