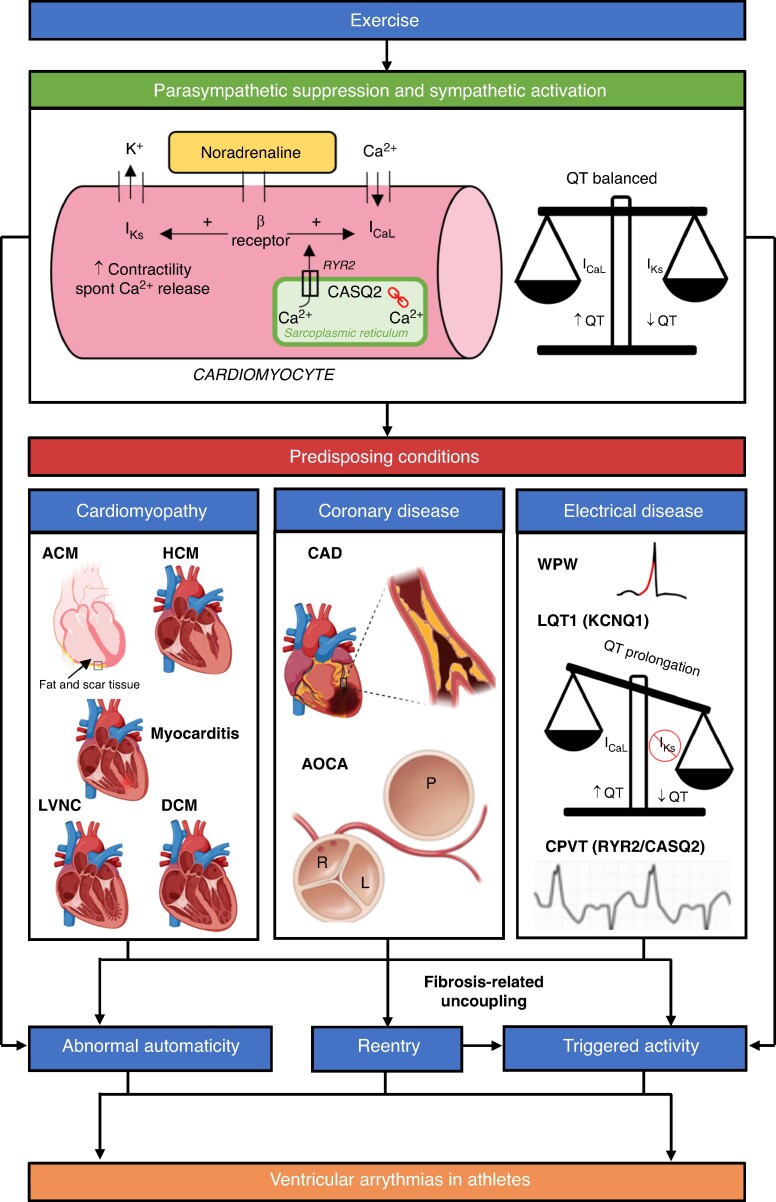
Figure 2.
A schematic of some of the proposed mechanisms for VAs in athletes. Exercise activates the sympathetic nervous system (SNS) and suppresses the parasympathetic nervous system. Binding of noradrenaline to cardiomyocytes and physiological adaptation of Ca2+ homeostasis promotes increased triggered activity. SNS activation also leads to abnormal automaticity. Concomitant activation of IKs ensures the QT is balanced. Increase in cardiac impulse formation via the above mechanisms can lead to idiopathic VAs in structurally normal hearts. Reentry is promoted by underlying myocardial fibrosis associated with cardiomyopathies and coronary anomalies. Some idiopathic VAs (e.g. LV fascicular VT) are also caused by reentry. Reentry itself can lead to triggered activity via fibrosis-related uncoupling. The combination of abnormal cardiac impulse formulation and reentry can lead to malignant VAs and SCD in athletes with predisposing conditions. Electrical diseases also cause VAs via other mechanisms. Long QT Syndrome type 1 (LQT1) syndrome is caused by pathological variants in the KCNQ1 gene whilst catecholaminergic polymorphic ventricular tachycardia (CPVT) is caused by pathogenic variants in Ryanodine Receptor 2 (RYR2, autosomal dominant, 50%) and Calsequestrin 2 (CASQ2, autosomal recessive, 2%) genes. Please note that this schematic does not include other proposed mechanisms for VAs in athletes such as stretch-activated ion channel expression and stretch-mediated gene expression. K+, potassium; Ca2+, calcium; β Receptor, β adrenergic receptor; IKs, slow delayed rectifier potassium channel; ICaL, L-type Ca2+ channel; ACM, arrhythmogenic cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVNC, left ventricular non-compaction cardiomyopathy; DCM, dilated cardiomyopathy; CAD, coronary artery disease; AOCA, anomalous origin of the coronary arteries; WPW, Wolff–Parkinson–White syndrome. Image created with BioRender.com.