Abstract
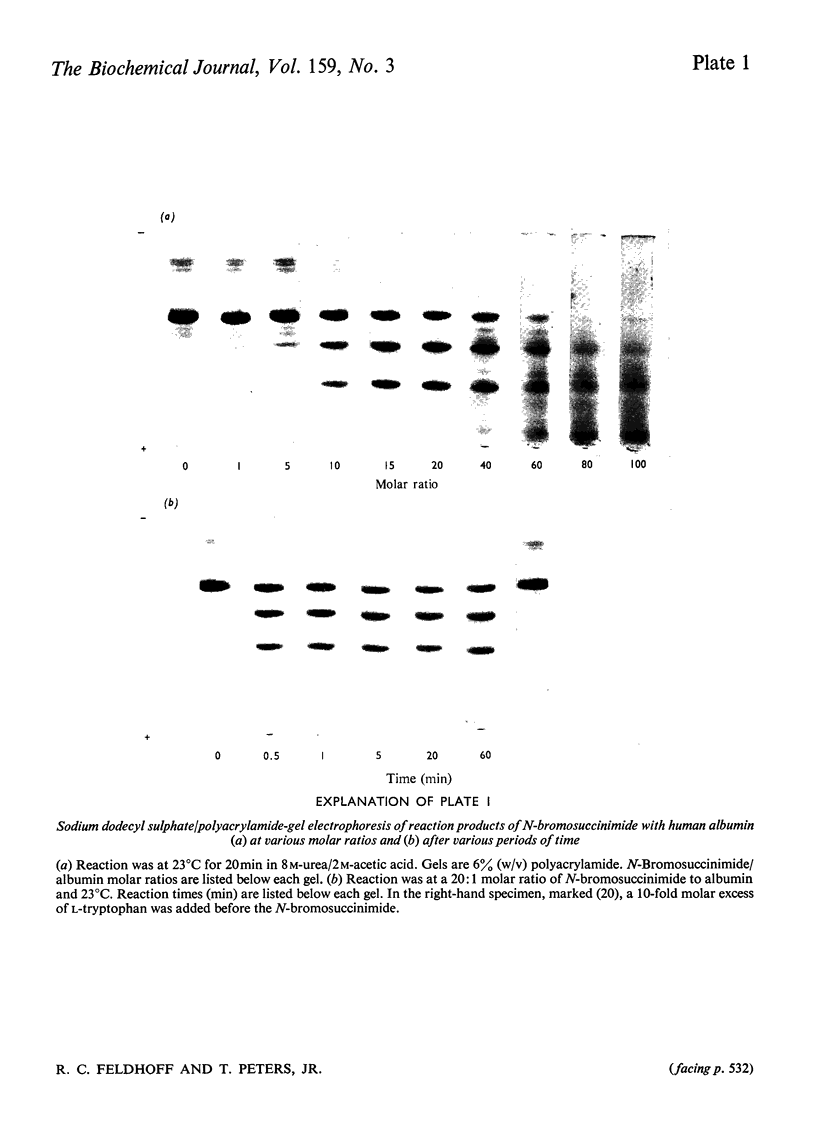
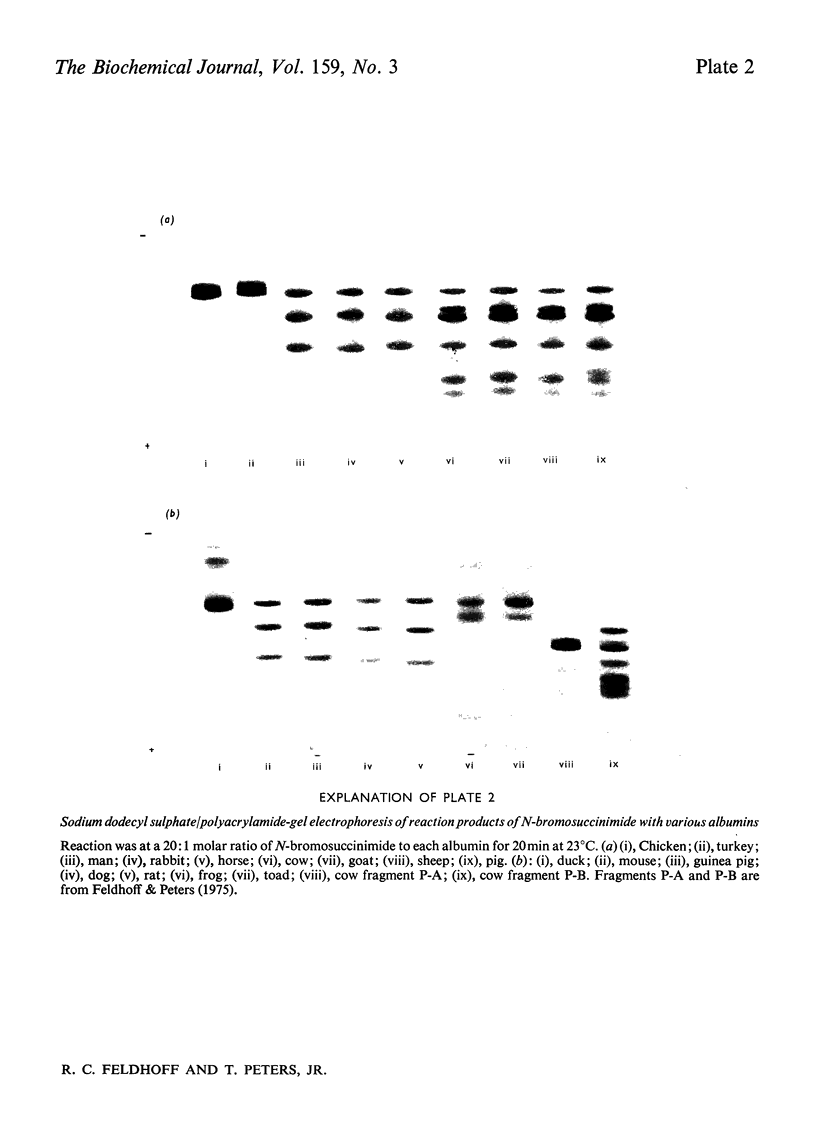
A technique is described by which both the numbers of tryptophan residues and their approximate locations in the peptide chain of a protein can be determined by cleavage with N-bromosuccinimide followed by polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate. The number of new peptide bands appearing in the gel is a function of the number of tryptophan residues, and the relative migration of the bands permits calculation of peptide molecular weights and an estimation of the positions of the tryptophan residues in the peptide chain. The technique uses a sample of about 0.5 mg and is suitable for any protein that contains a small number of tryptophan residues. These are the very specimens that are difficult to assay accurately for tryptophan by spectrophotometric or colorimetric methods. Tryptophan residues which are within about 20 residues of the ends of the peptide chain or of each other would not be detected. The specificity of the cleavage with N-bromosuccinimide was ascertained by utilizing human serum albumin, which is known to have a single tryptophan residue at position 214. The technique was then applied to a comparative study of the numbers and locations of tryptophans in the serum albumins of 16 species, namely 11 mammals, three birds and two amphibians. The number of tryptophan residues were confirmed by an independent colorimetric method. All of the mammalian albumins contained a tryptophan residue near position 213. The three avian albumins examined have no tryptophan. Frog and toad albumins contained two tryptophan residues, which appear to be situated at different positions from those in mammalian albumins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feldhoff R. C., Peters T., Jr Fragments of bovine serum albumin produced by limited proteolysis. Isolation and characterization of peptic fragments. Biochemistry. 1975 Oct 7;14(20):4508–4514. doi: 10.1021/bi00691a027. [DOI] [PubMed] [Google Scholar]
- Janatova J., Fuller J. K., Hunter M. J. The heterogeneity of bovine albumin with respect to sulfhydryl and dimer content. J Biol Chem. 1968 Jul 10;243(13):3612–3622. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meloun B., Morávek L., Kostka V. Complete amino acid sequence of human serum albumin. FEBS Lett. 1975 Oct 15;58(1):134–137. doi: 10.1016/0014-5793(75)80242-0. [DOI] [PubMed] [Google Scholar]
- Nagano H., Shimada T., Shukuya R. Further characterization of bullfrog serum albumin. Biochim Biophys Acta. 1973 Jun 15;310(2):495–499. doi: 10.1016/0005-2795(73)90134-7. [DOI] [PubMed] [Google Scholar]
- PETERS T., Jr Appearance of new N-terminal residues upon treatment of human and bovine serum albumin with N-bromosuccinimide. C R Trav Lab Carlsberg. 1959;31:227–234. [PubMed] [Google Scholar]
- PETERS T., LOGAN A. C., SANFORD C. A. Terminal amino acid residues of chicken, duck and turkey serum albumins. Biochim Biophys Acta. 1958 Oct;30(1):88–92. doi: 10.1016/0006-3002(58)90244-0. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Clin Chem. 1970;13:37–111. doi: 10.1016/s0065-2423(08)60385-6. [DOI] [PubMed] [Google Scholar]
- Reed R. G., Feldhoff R. C., Clute O. L., Peters T., Jr Fragments of bovine serum albumin produced by limited proteolysis. Conformation and ligand binding. Biochemistry. 1975 Oct 21;14(21):4578–4583. doi: 10.1021/bi00692a004. [DOI] [PubMed] [Google Scholar]
- Spies J. R. Determination of tryptophan in proteins. Anal Chem. 1967 Oct;39(12):1412–1416. doi: 10.1021/ac60256a004. [DOI] [PubMed] [Google Scholar]
- WITKOP B. Nonenzymatic methods for the preferential and selective cleavage and modification of proteins. Adv Protein Chem. 1961;16:221–321. doi: 10.1016/s0065-3233(08)60031-5. [DOI] [PubMed] [Google Scholar]
- Wallace D. G., Wilson A. C. Comparison of frog albumins with those of other vertebrates. J Mol Evol. 1972 Dec 29;2(1):72–86. doi: 10.1007/BF01653944. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]