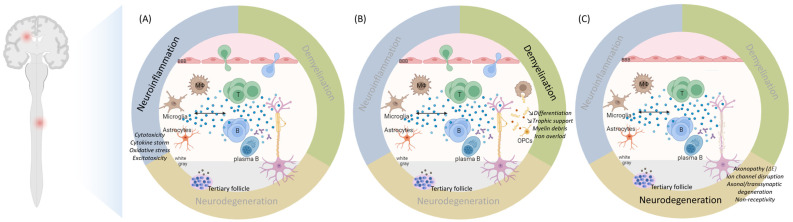
Figure 2.
The pathogenesis of multiple sclerosis. (A) Neuroinflammation is marked by the invasion of peripheral immune cells in the CNS through a disrupted BBB in the early inflammatory phase of MS. These cells are reactivated, secrete cytokines (e.g., IFNg by Th1, IL6/17 by Th17, GM-CSF, IL6, TNFa by B cells) and cytotoxic molecules (e.g., granzyme B by CD8+ T cells), attract more peripheral immune cells and activate macrophages, microglia and astrocytes, which produce cytokines, nitric oxide, and reactive oxygen species (ROS) (blue dots). B cells can also differentiate into autoantibody-producing plasma cells. With disease progression, infiltration of peripheral immune cells is reduced since the BBB is closed. CNS-resident cells, i.e., microglia and astrocytes, sustain the inflammation by producing cytokines (TNFa, IL6) and releasing ROS (blue dots). TNFa-mediated glutamate release from microglia and its impaired turnover by astrocytes result in excitotoxicity. Remarkably, plasmablasts and plasma B cells form tertiary follicle-like structures in the meninges that may release proinflammatory factors activating microglia (brown dots). (B) Demyelination is partly caused by this cytotoxic and proinflammatory environment that breaks down the myelin sheaths. Macrophages and microglia, attracted by astrocytes, can clear the myelin debris, allowing (partial) remyelination by surviving oligodendrocytes or by OPCs proliferating, migrating, and differentiating at the site of injury in response to cytokines, chemokines (CXCL1, CXCL12), mitogens (platelet-derived growth factor), chemoattractants (semaphorin 3F), and trophic factors (insulin-like growth factor, ciliary neurotrophic factor) (blue dots) released by microglia and astrocytes. This will reduce the harm to the axons. However, the phagocytic capacity of microglia/macrophages decreases with disease progression. Hence, myelin debris are improperly cleared, trigger an inflammatory response, and inhibit axonal growth, and OPCs are less recruited and fail to differentiate. The trophic support of oligodendrocytes to the underlying axons wanes. The ferrous iron (red dots) released from the myelin, where it accumulates with age, is oxidized to ferric iron, producing ROS and causing lipid peroxidation and ferroptosis. (C) Neurodegeneration starts early in the disease and becomes prominent with disease progression when the compensatory mechanisms safeguarding the CNS reserve are exceeded. Axons are directly harmed by the proinflammatory and oxidative environment, but also by the loss of the insulating and supporting myelin sheaths. Chronically demyelinated axons seem to be non-receptive to OPCs. Nodal and paranodal ion channels are disorganized; synapses are dysfunctional. Axons suffer a major energy debt altering axonal transport of mitochondria and synaptic vesicles. The axonopathy spreads the axonal and transsynaptic degeneration. These events are self-sustained and intermingled, further enhanced by senescent processes, resulting in a major cytokine storm and oxidative burst, mitochondrial dysfunction, mitochondrial DNA damage, energy failure, ion imbalance, cytotoxicity, excitotoxicity, lack of trophic support by the loss of oligodendrocytes, and axonal loss. BBB = blood–brain barrier; B = B cell; CNS = central nervous system; ΔE = energy deficit: MS = multiple sclerosis; OPC = oligodendrocyte progenitor cell; ROS = reactive oxygen species; T = T cell; Th = T helper cell. Created in BioRender.com.