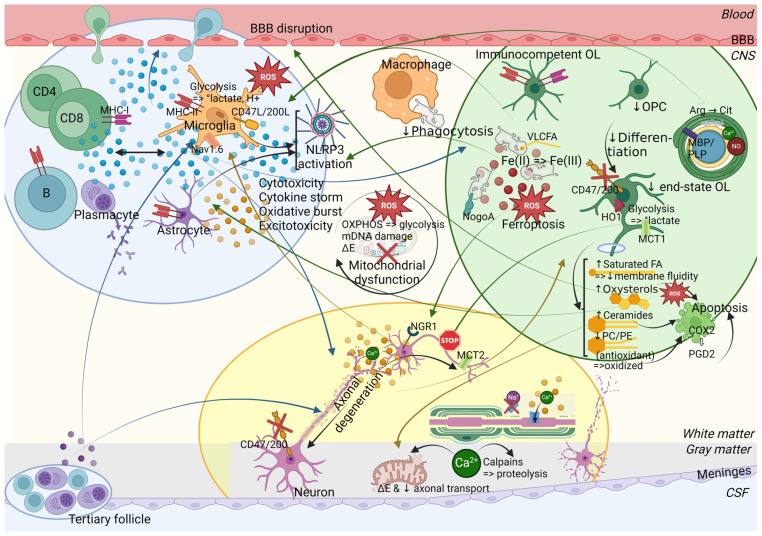
Figure 4.
Pathophysiological mechanisms involved in the triangulation of neuroinflammation (blue bubble), demyelination (green bubble), and neurodegeneration (yellow bubble) in MS. The impact of each on the other processes is indicated by arrows in the corresponding color, whereas intrinsic effects are indicated by black arrows. Neuroinflammation (blue bubble): Peripheral immune cells and CNS-resident cells attract and activate each other through the release of cytokines and chemokines (blue dots), whereby MHC type I and II play an important role in antigen presentation. Cytokines also contribute to the disruption of the BBB. CD8+ T cells, reactive microglia, and astrocytes cause cytotoxicity. Plasma B cells release antibodies of partially unknown significance, which may contribute to the pathophysiology via antibody-dependent cellular toxicity, opsonization, complement-dependent cytotoxicity, and antibody-induced demyelination. The activation of microglia (which relies on glycolysis and lactate production with extracellular acidification) and astrocytes results in the production of ROS. Microgliosis and astrogliosis also involve NLRP3 inflammasome activation. The resulting proinflammatory and oxidative environment harms OPCs, oligodendrocytes, and neurons. High levels of glutamate (orange dots) (due to TNFa/ATP-induced release from microglia and reduced turnover by astrocytes) cause axonal/neuronal and oligodendroglial damage through excitotoxicity via NMDA receptors. Meningeal inflammation mainly consists of plasmablasts and plasma B cells, which may form tertiary follicle-like structures. It is correlated with subpial demyelination and CSF protein levels of TNF, IFNg, and CXCL13. Thus, they affect neurons directly or by releasing an unknown soluble factor (purple dots) that activates microglia. The CD47 and CD200 expressed on oligodendrocytes and neurons inhibit their phagocytosis by microglia by binding to CD47L and CD200L (“Don’t eat me” signal) but the former are downregulated. Demyelination (green bubble): Subclusters of OPCs and end-state oligodendrocytes are reduced, as well as the recruitment, proliferation, and differentiation of OPCs into oligodendrocytes. Myelin defects appear to begin at the inner layer and are caused by (i) the citrullination of MBP (i.e., the conversion of positively charged arginine (Arg) into uncharged citrulline (Cit)) altering the interaction of MBP with the plasma membrane, (ii) MBP phase transition resulting from increased calcium levels and causing myelin vesiculation, and (iii) protein S-nitrosylation, especially of PLP, caused by nitric oxide (NO). Moreover, the lipid species in the plasma membrane are altered, as evidenced by an increase in saturated fatty acids, which reduces membrane fluidity, an increase in oxysterols and ceramides, and a decrease in phosphatidylcholine (PC) and phosphatidylethanolamine (PE), which have antioxidant properties. Oxysterols (by inducing oxidative stress), ceramides and the oxidized forms of PC and PE cause oligodendrocyte apoptosis, which results in the expression of COX2 that mediates the production of proinflammatory prostaglandins (e.g., PGD2), thereby sustaining apoptosis. Oligodendrocyte apoptosis further induces gliosis, saturated fatty acids can promote microglial activation, while ceramides enhance Th1 cytokine production and cause mitochondrial dysfunction in neurons. Moreover, citrullinated MBP is an immunogenic trigger, and immunocompetent oligodendrocytes express MHC-I/II and interferon-responsive genes. OPC support to the BBB is decreased, and BBB disruption is further enhanced by oxysterols. Myelin debris are initially cleared by macrophages/microglia; however, their phagocytic capacity decreases with disease progression and cellular senescence. Uncleared myelin debris induce NLRP3 inflammasome activation in microglia. They halt OPC differentiation and expose myelin-associated inhibitory factors, such as reticulon 4, previously known as neurite outgrowth inhibitory factor (NogoA), as well as myelin-associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMG), which bind to the Nogo receptor 1 (NGR1) expressed on axons, thereby inhibiting axonal growth and regeneration. They are also enriched in very long-chain fatty acids (VLCFA) by impaired beta oxidation in peroxisomes, which contributes to neurotoxicity and neuroinflammation. Myelin stores iron, which increases with age, but causes ferroptosis when the storage capacity of ferritin is exceeded. The released ferrous iron (Fe(II)) is oxidized to ferric iron (Fe(III)), resulting in the production of hydroxyl radicals. Microglial uptake of iron causes their dystrophy resulting in a second release of iron. Furthermore, oligodendrocytes express heme oxygenase 1 (HO1) via the nuclear factor erythroid 2-related (NRF2) pathway in response to oxidative stress, resulting in the production of ferrous iron (Fe(II)) and bilirubin, an antioxidant, in an attempt to counter it; however, this also forms an additional source of harmful ferrous iron. Finally, oligodendrocytes safeguard the trophic support of axons by the exchange of lactate (produced by glycolysis in oligodendrocytes) through monocarboxylate transporter 1 (MCT1, expressed in oligodendrocytes) and MCT2 (expressed in neurons); however, this lactate supply is impaired, resulting in an axonal energy deficit. Neurodegeneration (yellow bubble): Axonopathy results from mitochondrial dysfunction, ion imbalance (by nodal/paranodal disruption of ion channels linked to demyelination with loss of saltatory conduction), and energy deficit. Intracellular calcium overload, entering via the sodium/calcium exchanger in reverse mode, the mitochondrial calcium uniporter, and acid-sensing ion channels (activated by inflammation-linked tissue acidosis), and enhanced by glutamate, plays a key role in inducing calpains, which are proteases that degrade the cytoskeleton and reduce axonal transport. Moreover, mitochondrial permeability transition pores are formed when intracellular calcium levels increase and cause the leakage of mitochondrial solutes resulting in mitochondrial collapse. Axonopathy causes anterograde and retrograde axonal and transsynaptic degeneration. Cortical demyelination and neuronal apoptosis can result in brain atrophy. Chronically demyelinated neurons appear to be unreceptive to myelin expansions of differentiating oligodendrocytes and to express fewer growth factors supporting oligodendrocytes. Moreover, OPCs sense axonal synaptic dysfunction within neuron-to-OPC synapses. Finally, neurons can induce microglia by the release of ATP and ion imbalance (Nav1.6 expressed on microglia). Mitochondrial dysfunction is enhanced by oxidative stress (increased ROS/RNS, reduced antioxidant NRF2 pathway), which causes mitochondrial DNA (mDNA) damage (deletions), oxidizes lipids and proteins, and alters the mitochondrial respiratory chain (also due to mitochondrial DNA deletions). There is a metabolic shift from oxidative phosphorylation (OXPHOS) to glycolysis to allow a rapid ATP production despite its relative inefficiency. This results in virtual hypoxia and energy failure. Neurons (by reduced PPARGC1A expression, regulating mitochondrial function) and OPCs/oligodendrocytes (by their iron storage and reduced antioxidant mechanisms) are particularly vulnerable. BBB = blood–brain barrier, CNS = central nervous system, CSF = cerebrospinal fluid, ΔE = energy deficit, MHC-I/II = major histocompatibility complex type I or II, MS = multiple sclerosis, OPC = oligodendrocyte progenitor cell, OL = oligodendrocyte, RNS = reactive nitrogen species, ROS = reactive oxygen species, ↑ = increased, ↓ = decreased. Created in BioRender.com.