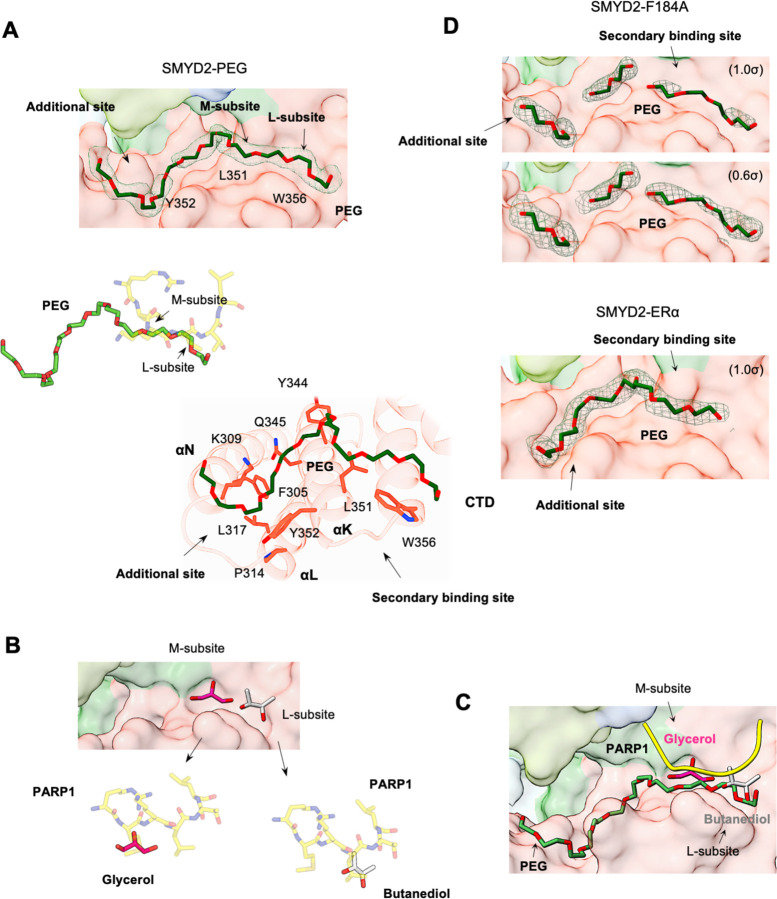
Fig. 3. Promiscuous secondary binding site.
(A) The PEG binding site in the SMYD2-PEG structure. Surface representation of the PEG binding site (top), superposition of PEG and the PARP1 peptide (middle), and SMYD2 residues involved in PEG binding (bottom). PEG, the PARP1 peptide, and SMYD2 residues are depicted as sticks with carbon atoms colored in dark green, yellow, and red, respectively. Residues important for forming the secondary binding site, including L351, Y352, and W356, are labeled on the surface. (B) Butanediol (grey) and glycerol (magenta) bound at the secondary binding site and their superposition with the PARP1 peptide. (C) Promiscuous secondary binding site overlaid with the PARP 1 peptide (yellow), PEG (dark green), butanediol (grey), and glycerol (magenta). (D) The PEG binding site in the SMYD2-F184A and SMYD2-ERα structures. 2Fo – Fc omit maps of PEG molecules were contoured at 1.0 σ for SMYD2-PEG and SMYD2-ERα, and 1.0 σ and 0.6 σ for SMYD2-F184A.