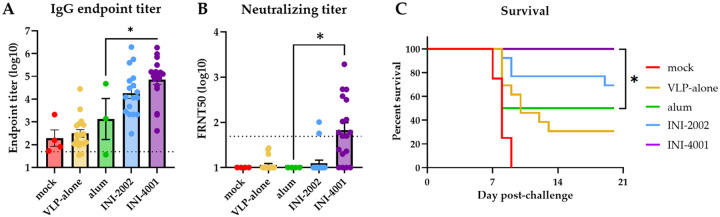
Fig 2. INI-4001-adjuvanted VLP elicits superior antibody response and protection from lethal POWV challenge.
(A-B) Mice prime-boost vaccinated 2 weeks apart sc with 106 FFUe of VLP alone or adjuvanted with 300μg alum, 1nmol INI-2002, or 10nmol INI-4001. Mock group injected with PBS vehicle alone. mock n=4; VLP-alone n=18–19; alum n=3–4; INI-2002 n=18; INI-4001 n=18. Serum collected 2 weeks post-boost and analyzed by whole-virus ELISA (A) or FRNT (B). Data are reported as log-transformed mean endpoint titer ± SEM for IgG titer and reciprocal FRNT50 for neutralizing titer. Dotted line represents least dilute sera tested (1:50). Data represent three independent experiments. (A) Statistical significance determined by one-way ANOVA and Šídák’s multiple comparisons test to compare treatments to alum group after log transformation. * = p < 0.05. (B) Statistical significance determined by one-way ANOVA and Šídák’s multiple comparisons test to compare alum group to INI-2002 and INI-4001. (C) Vaccinated mice challenged with a lethal 104 FFU dose of POWV-LB 2 weeks post-boost. Survival of mice assessed to day 20 post-challenge. mock n=4; VLP-alone n=13; alum n=4; INI-2002 n=13; INI-4001 n=13. Data represent three independent experiments. Statistical significance determined by log rank test with Bonferroni correction to compare adjuvant groups to VLP-alone. * = p < 0.05.