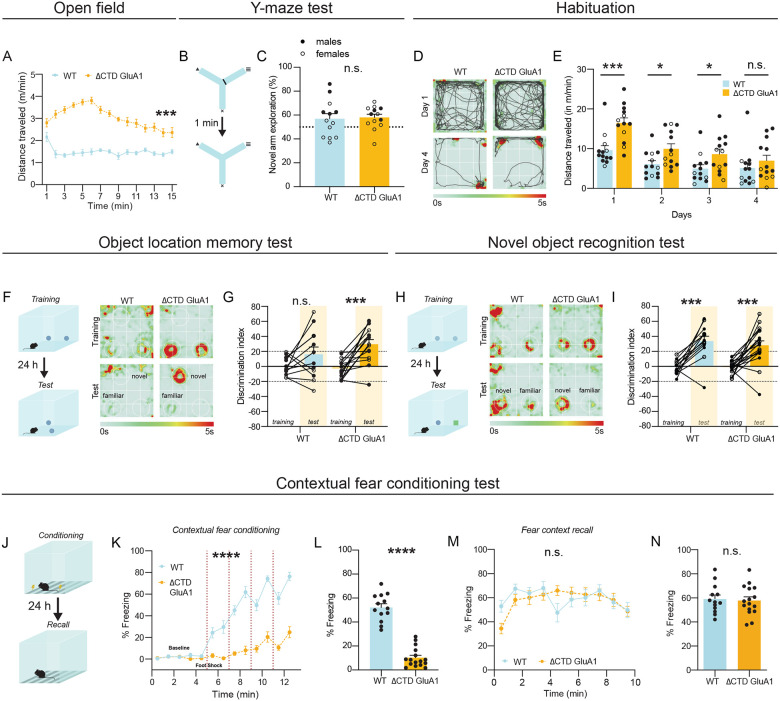
Figure 6. Intact excitatory synaptic transmission and LTP in DG granule cells but altered excitatory synaptic transmission in DG inhibitory INs in ΔCTD GluA1 mice.
A: Whole-cell patch-clamp recording set-up for slice electrophysiology experiments in DG granule cells (GCs). B: Average paired-pulse ratio (PPR) values for evoked AMPAR EPSCs in WT and ΔCTD GluA1 GCs. Representative WT (blue) and ΔCTD GluA1 (yellow) traces are shown to the right of the plot. C: Average AMPAR/NMDAR ratios in WT and ΔCTD GluA1 GCs. D: Input-output relationship plot of AMPAR EPSCs in WT and ΔCTD GluA1 DG GCs. Representative WT (blue) and ΔCTD GluA1 (yellow) traces are shown to the right of the plot. E: AMPAR EPSC amplitude of WT and ΔCTD GluA1 DG GCs normalized to the mean AMPAR EPSC amplitude before theta-burst LTP induction (arrow). Representative WT (blue) and ΔCTD GluA1 (yellow) traces are shown to the right of the plot. n indicates number of cells induced / number of cells at the end of the experiment (min. 40). F: Whole-cell patch-clamp recording set-up for slice electrophysiology experiments in DG INs. WT and ΔCTD GluA1 mice were stereotaxically injected (AAV-mDLX-GFP) to label INs in DG. G: Mean values of AMPAR/NMDAR ratios in WT and ΔCTD GluA1 mDLX-GFP(+)-labelled INs. Representative WT (blue) and ΔCTD GluA1 (yellow) traces are shown to the right of the plot. Error bars represent SEM. Scale bars: 50pA, 20ms. n.s., not statistically different; *, p≤0.05. B-C, E, G: unpaired t-test. D: two-way ANOVA.