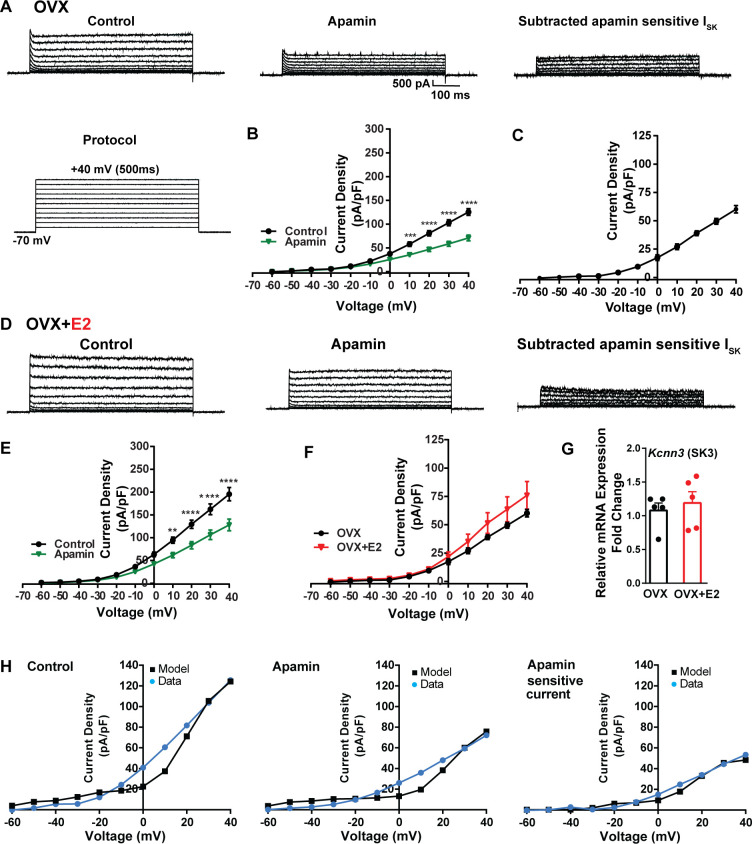
Figure 7. Contribution of small conductance, calcium-activated K+ (SK) channel to Kiss1ARH neuronal excitability.
(A) Representative traces of the inhibition of outward currents before (left, control) and after the specific SK blocker apamin (500 nM, middle). Apamin-sensitive currents were calculated from the subtraction of control and apamin at depolarized potentials (right). Cells were clamped at –70 mV and given 500 ms voltage pulses from –60 mV to +40 mV in 10 mV steps at 0.2 Hz, as shown in (A) at the bottom. (B) Mean current density–voltage relationships measured at the end of the 500 ms voltage step ranging from –60 mV to +40 mV were obtained in the absence and presence of apamin (two-way ANOVA: main effect of treatment [F(1, 8) = 22.69, p=0.0014], main effect of voltage [F(10, 80) = 306.0, p<0.0001] and interaction [F(10, 80) = 24.76, p<0.0001]; mean ± SEM; n = 5; post hoc Bonferroni test, ***p<0.005, ****p<0.001). (C) Apamin-sensitive current densities were obtained from (B) (mean ± SEM, n = 5). (D) Representative traces of the inhibition of outward currents before (left, control) and after the specific SK blocker apamin (500 nM, middle). Apamin-sensitive currents resulted from the subtraction of control and apamin at depolarized potentials (right). (E) Mean current density–voltage relationships measured at the end of the 500 ms voltage step ranging from –60 mV to +40 mV were obtained in the absence and presence of apamin (two-way ANOVA: main effect of treatment [F(1, 10) = 12.85, p=0.0050], main effect of voltage [F(10,100) = 264.1, p<0.0001] and interaction [F(10, 100)=11.93, p<0.0001]; mean ± SEM, n = 6; post hoc Bonferroni test, **p<0.01, ****p<0.001). (F) Apamin-sensitive current densities were obtained from (C) and (E) (ns; two-way ANOVA followed by Bonferroni post hoc test; mean ± SEM; OVX, n = 5; OVX + E2, n = 6). (G) Kiss1ARH neurons (three 10-cell pools) were harvested from each of five vehicle- and five E2-treated, OVX females to quantify the mRNA expression of Kcnn3 ion channel. E2 did not increase the mRNA expression of small conductance calcium-activated K+ (SK3) channels in Kiss1ARH. Bar graphs represent the mean ± SEM, with data points representing individual animals, oil versus E2, Unpaired t-test, t(8) = 0.551, p=0.5967. The expression values were calculated via the ΔΔCT method, normalized to Gapdh and relative to the oil control values. (H) The mathematical model was calibrated on the electrophysiology data from Kiss1ARH neurons before and after treatment with the specific SK blocker apamin, left panel versus middle panel, respectively. For the calibration, it was assumed that the applied concentration of apamin (500 nM) completely blocked the SK current. The modeled apamin-sensitive current with gSK = 28.1 nS (right panel) matches the electrophysiological data from OVX animals.