Abstract
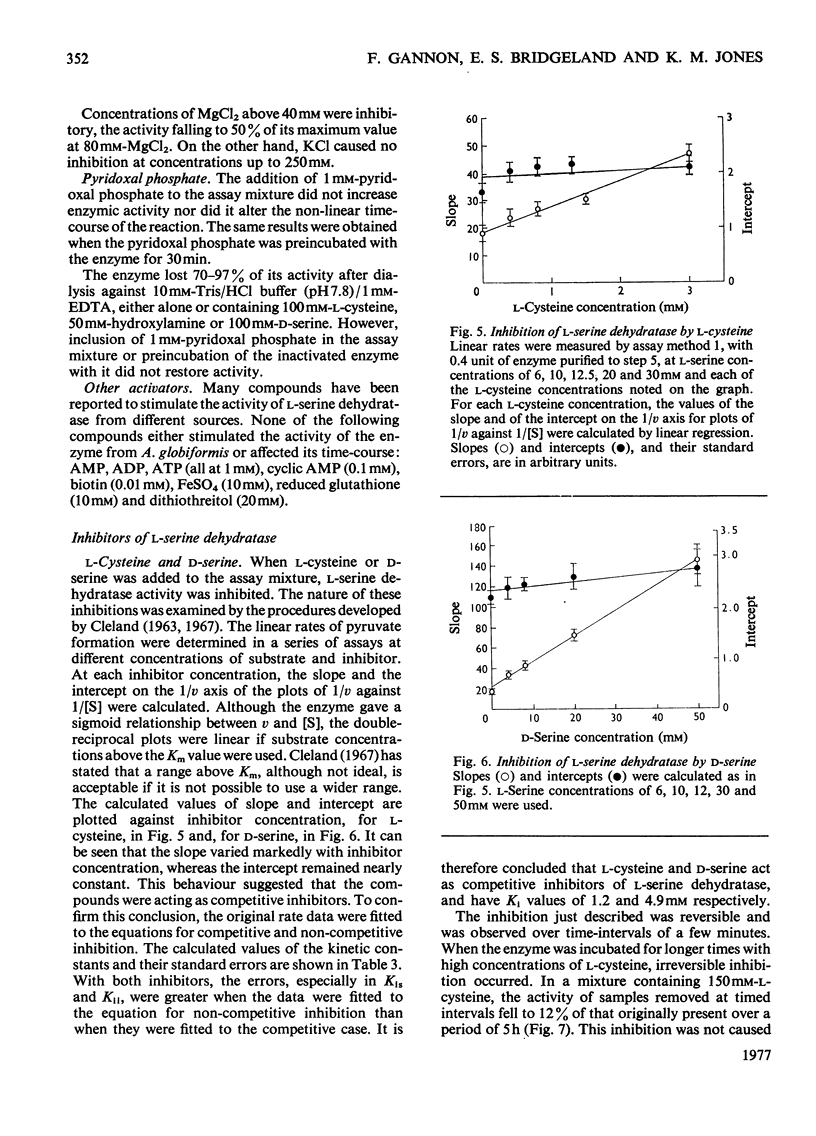
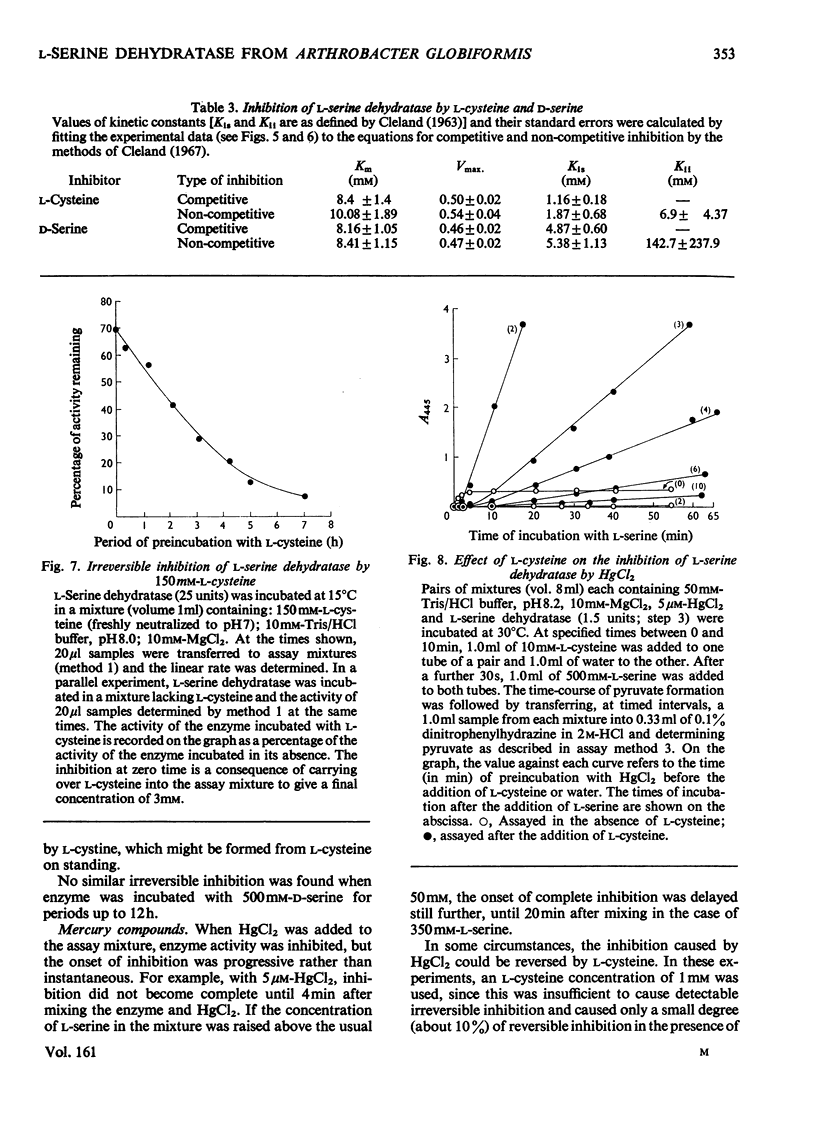
1. L-Serine dehydratase (EC 4.2.1.13) was purified 970-fold from glycine-grown Arthrobacter globiformis to a final specific activity of 660micronmol of pyruvate formed/min per mg of protein. 2. The enzyme is specific for L-serine; D-serine, L-threonine and L-cysteine are not attacked. 3. The time-course of pyruvate formation by the purified enzyme, in common with enzyme in crude extracts and throughout the purification, is non-linear. The reaction rate increases progressively for several minutes before becoming constant. The enzyme is activated by preincubation with L-serine and a linear time-course is then obtained. 4. The substrate-saturation curve for L-serine is sigmoid. The value of [S]0.5 varies with protein concentration, from 6.5mM at 23microng/ml to 20mM at 0.23microng/ml. The Hill coefficient remains constant at 2.9.5 The enzyme shows a non-specific requirement for a univalent or bivalent cation. Half-maximal activity is produced by 1.0mM-MgCl2 or by 22.5mM-KCl. 6. L-Cysteine and D-serine act as competitive inhibitors of L-serine dehydratase, with Ki values of 1.2 and 4.9mM respectively. L-Cysteine, at higher concentrations, also causes a slowly developing irreversible inhibition of the enzyme. 7. Inhibition by HgCl2 (5micronM)can be partially reversed in its initial phase by 1mM-L-cysteine, but after 10 min it becomes irreversible. 8. In contrast with the situation in all cell-free preparations, toluene-treated cells of A. globiformis form pyruvate from L-serine at a constant rate from the initiation of the reaction, show a hyperbolic substrate-saturation curve with an apparent Km of 7mM and do not require a cation for activity.
Full text
PDF


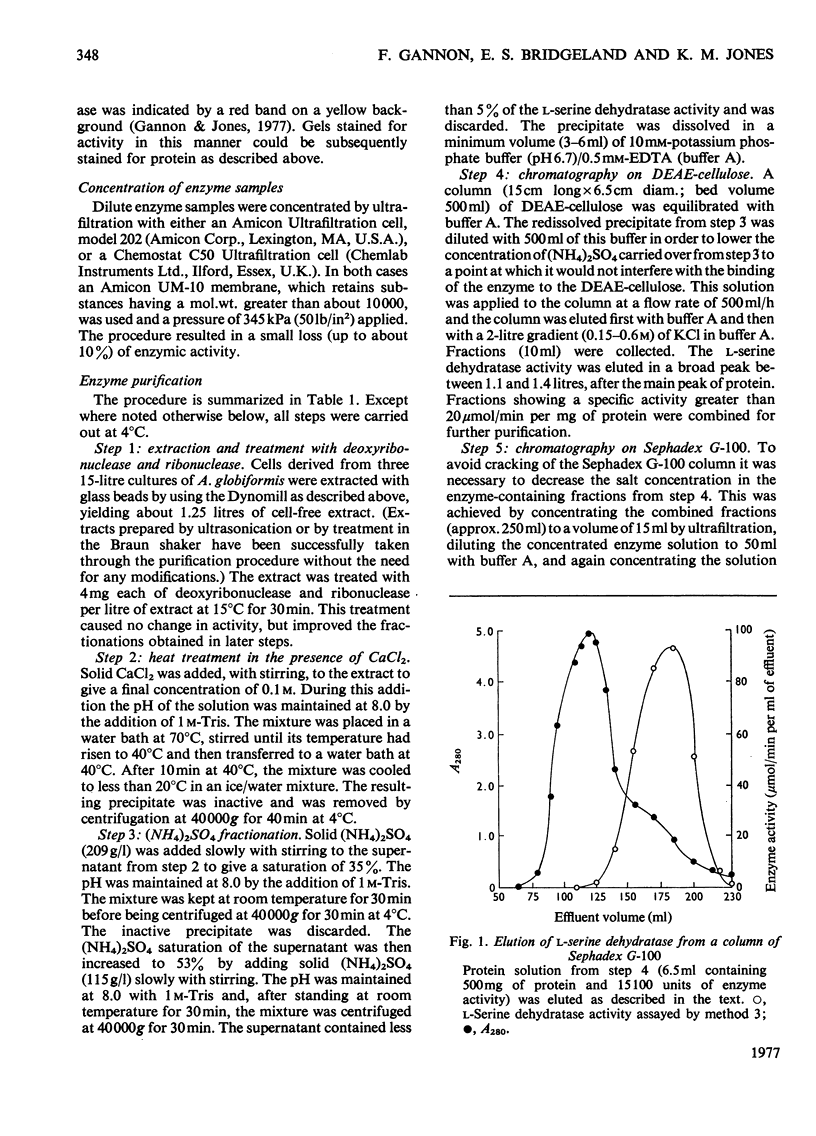
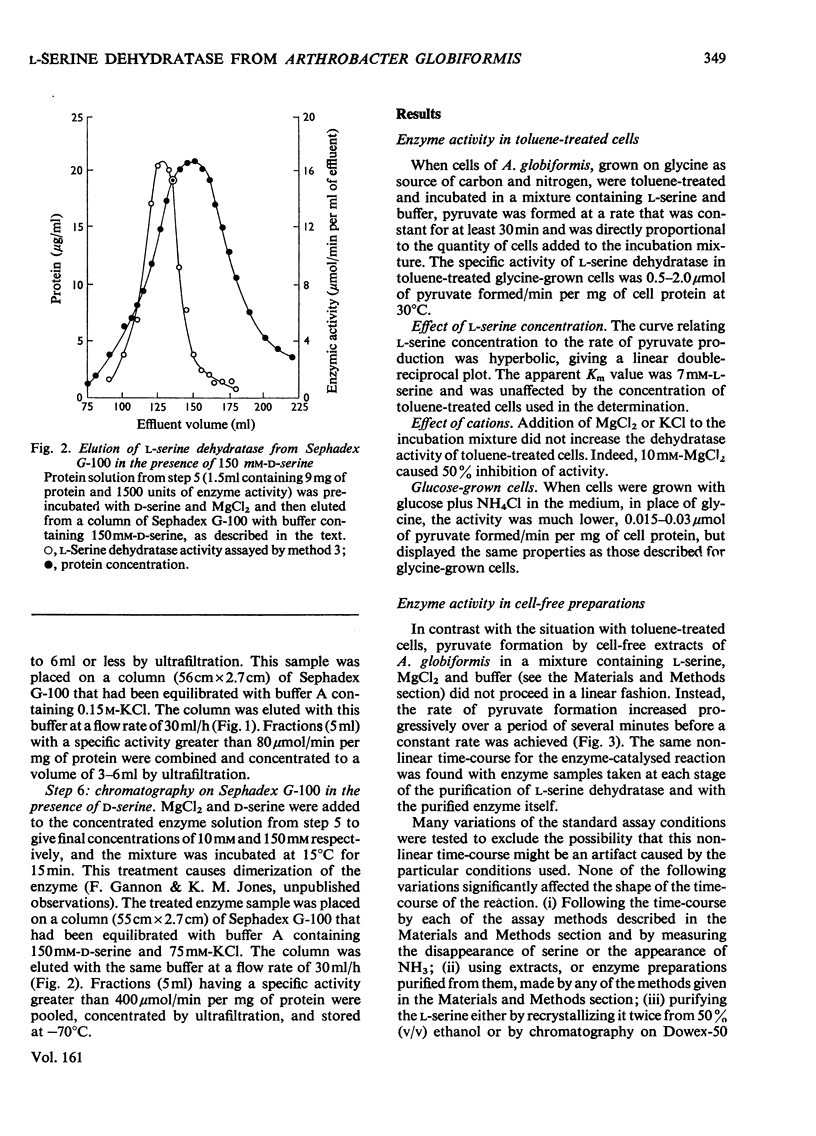
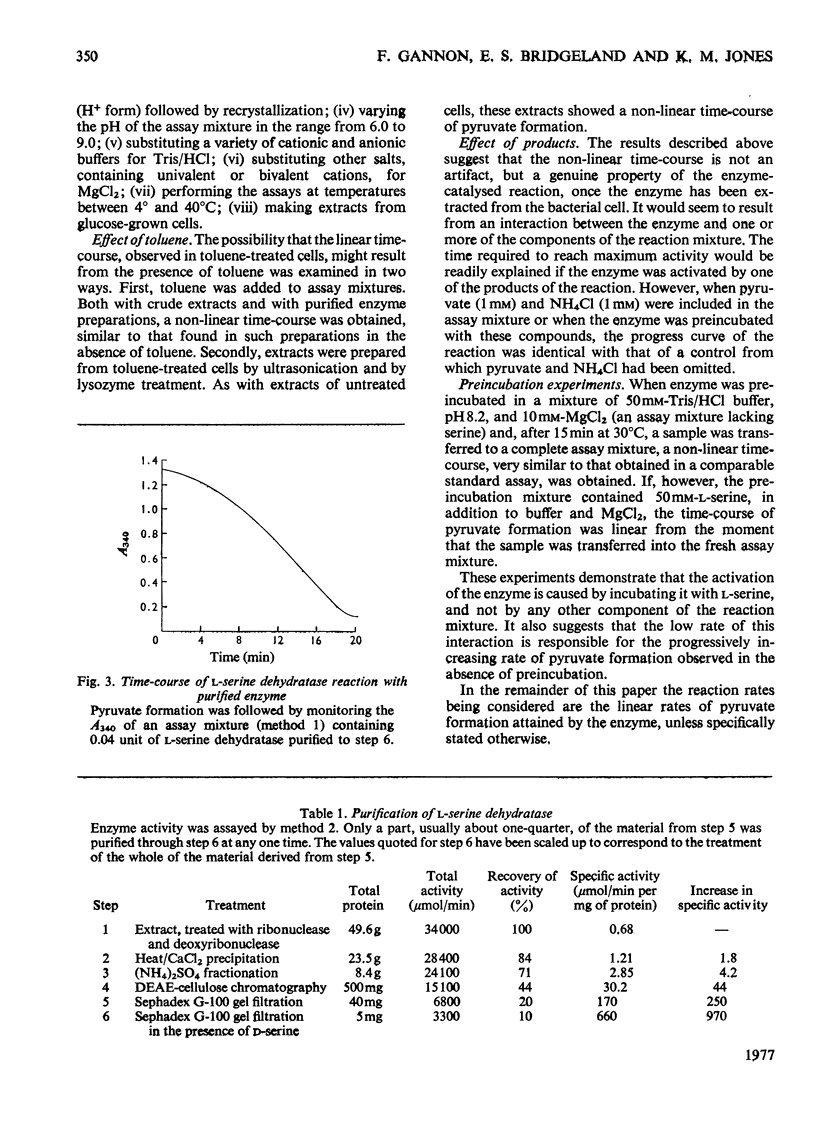
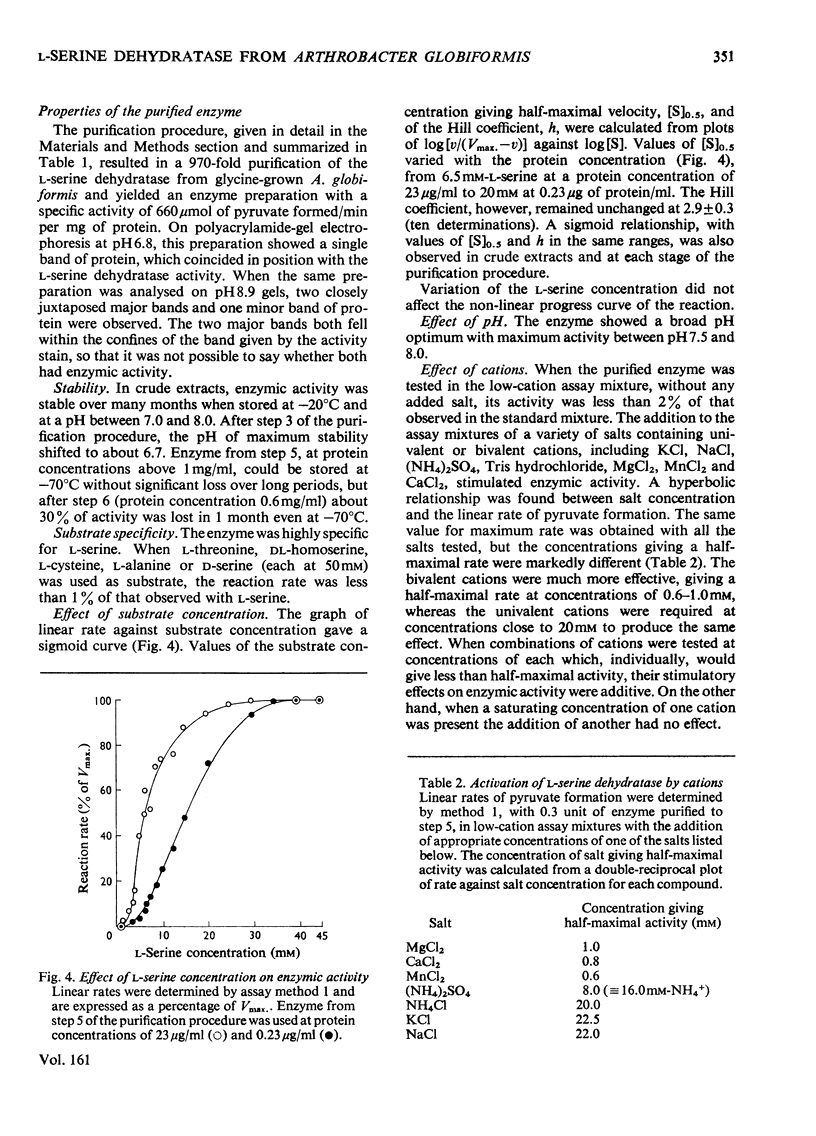


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alföldi L., Raskó I., Kerekes E. L-serine deaminase of Escherichia coli. J Bacteriol. 1968 Nov;96(5):1512–1518. doi: 10.1128/jb.96.5.1512-1518.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- CRAWFORD I. P., ITO J. SERINE DEAMINATION BY THE B PROTEIN OF ESCHERICHIA COLI TRYPTOPHAN SYNTHETASE. Proc Natl Acad Sci U S A. 1964 Mar;51:390–397. doi: 10.1073/pnas.51.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. E., Sagers R. D. Ferrous ion-dependent L-serine dehydratase from Clostridium acidiurici. J Bacteriol. 1972 Feb;109(2):757–763. doi: 10.1128/jb.109.2.757-763.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dowhan W., Jr, Snell E. E. D-serine dehydratase from Escherichia coli. 3. Resolution of pyridoxal 5'-phosphate and coenzyme specificity. J Biol Chem. 1970 Sep 25;245(18):4629–4635. [PubMed] [Google Scholar]
- Dowhan W., Jr, Snell E. E. D-serine dehydratase from Escherichia coli. II. Analytical studies and subunit structure. J Biol Chem. 1970 Sep 25;245(18):4618–4628. [PubMed] [Google Scholar]
- FRISELL W. R., MEECH L. A., MACKENZIE C. G. A simplified photometric analysis for serine and formaldehyde. J Biol Chem. 1954 Apr;207(2):709–716. [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Griffiths S. K., DeMoss R. D. Physiological comparison of L-serine dehydratase and tryptophanase from Bacillus alvei. J Bacteriol. 1970 Mar;101(3):813–820. doi: 10.1128/jb.101.3.813-820.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer H., Cennamo C., Boll M. Product activation of yeast threonine dehydratase by ammonia. Biochem Biophys Res Commun. 1964;14:487–492. doi: 10.1016/0006-291x(64)90256-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NAGABHUSHANAM A., GREENBERG D. M. ISOLATION AND PROPERTIES OF A HOMOGENEOUS PREPARATION OF CYSTATHIONINE SYNTHETASE-L-SERINE AND L-THREONINE DEHYDRATASE. J Biol Chem. 1965 Jul;240:3002–3008. [PubMed] [Google Scholar]
- Orr M. D., Blakley R. L., Panagou D. Discontinuous buffer systems for analytical and preparative electrophoresis of enzymes on polyacrylamide gel. Anal Biochem. 1972 Jan;45(1):68–85. doi: 10.1016/0003-2697(72)90008-5. [DOI] [PubMed] [Google Scholar]
- Raskó I., Kerekes E., Alföldi L. Properties of L-serine deaminase from Salmonella typhi-murium and Bacillus cereus. Acta Microbiol Acad Sci Hung. 1969;16(3):237–244. [PubMed] [Google Scholar]
- Reeves R. E., Sols A. Regulation of Escherichia coli phosphofructokinase in situ. Biochem Biophys Res Commun. 1973 Jan 23;50(2):459–466. doi: 10.1016/0006-291x(73)90862-0. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Threonine deaminases. Adv Enzymol Relat Areas Mol Biol. 1973;37:349–395. doi: 10.1002/9780470122822.ch6. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D. Behaviour of enzymes at high concentration. Use of permeabilised cells in the study of enzyme activity and its regulation. FEBS Lett. 1973 Jun 1;32(2):247–250. doi: 10.1016/0014-5793(73)80843-9. [DOI] [PubMed] [Google Scholar]


