Figure 2. In vivo efficacy of VIR-7229.
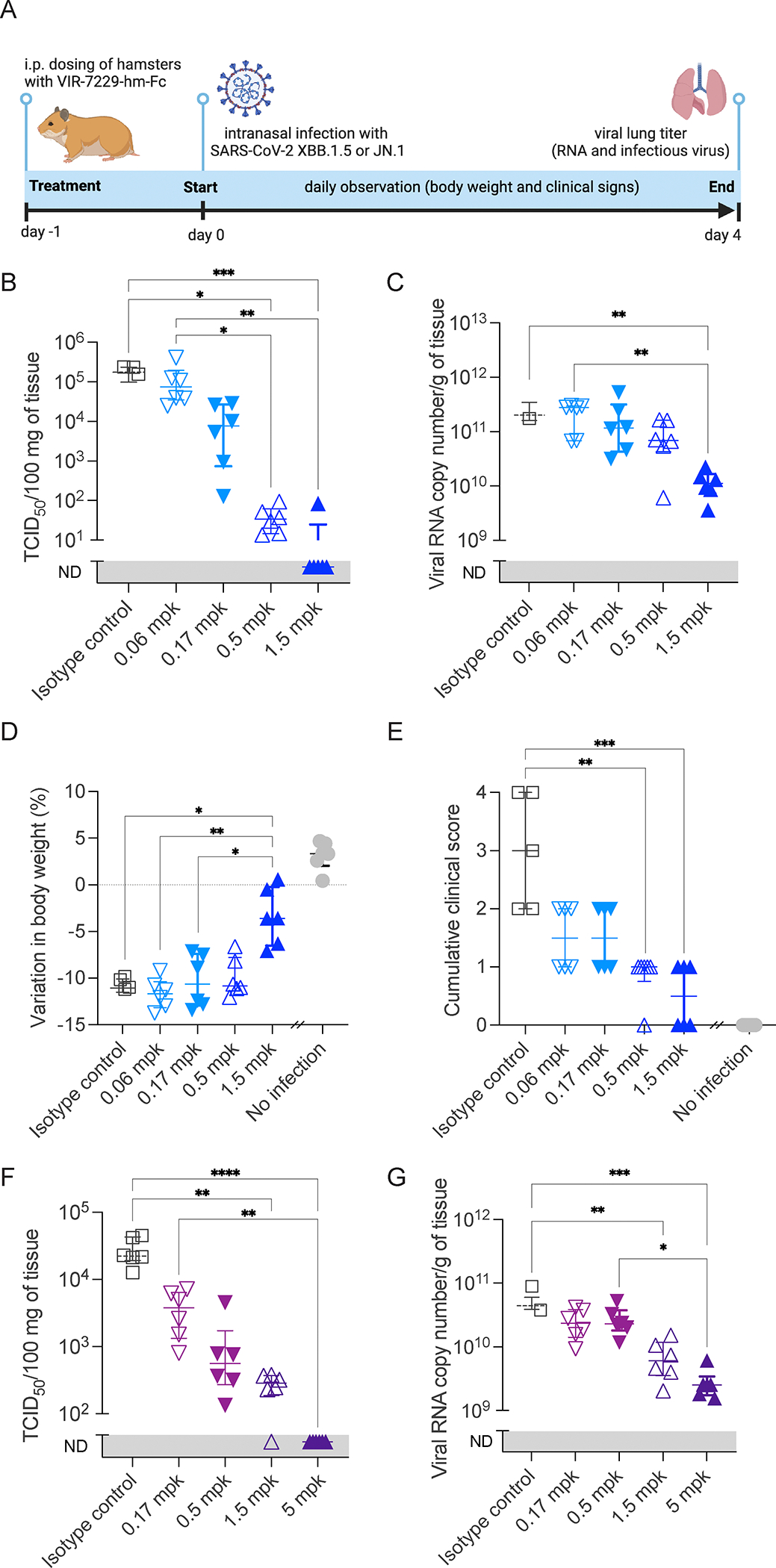
Virology and clinical endpoints on day 4 after SARS-CoV-2 XBB.1.5 or JN.1 infection of Syrian hamsters prophylactically administered with VIR-7229 (hamster Fc) or 1.5 mg/kg (mpk) isotype-matched control antibody. See also Data S3.
(A) Experiment outline.
(B) Infectious viral lung titers for XBB.1.5 infection. ND: not detected.
(C) Lung viral RNA load for XBB.1.5 infection. ND: not detected.
(D) Variation in body weight relative to day 0 for XBB.1.5 infection. No-infection control from the JN.1 experiment is provided for qualitative comparison.
(E) Cumulative clinical score for XBB.1.5 infection (0–4): ruffled fur, slow movements, apathy, absence of exploratory activity. No-infection control from the JN.1 experiment is provided for qualitative comparison.
(F) Infectious viral lung titers for JN.1 infection.
(G) Lung viral RNA load for JN.1 infection. ND: not detected.
(B-G) X-axis indicates dose of VIR-7229-hmFc or 1.5 mpk isotype control. Median ± interquartile range is shown; significance is based on ANOVA non-parametric Kruskal-Wallis test followed by Dunn’s multiple comparison test, *p<0.05, **p< 0.01, *** p < 0.001.
