Abstract
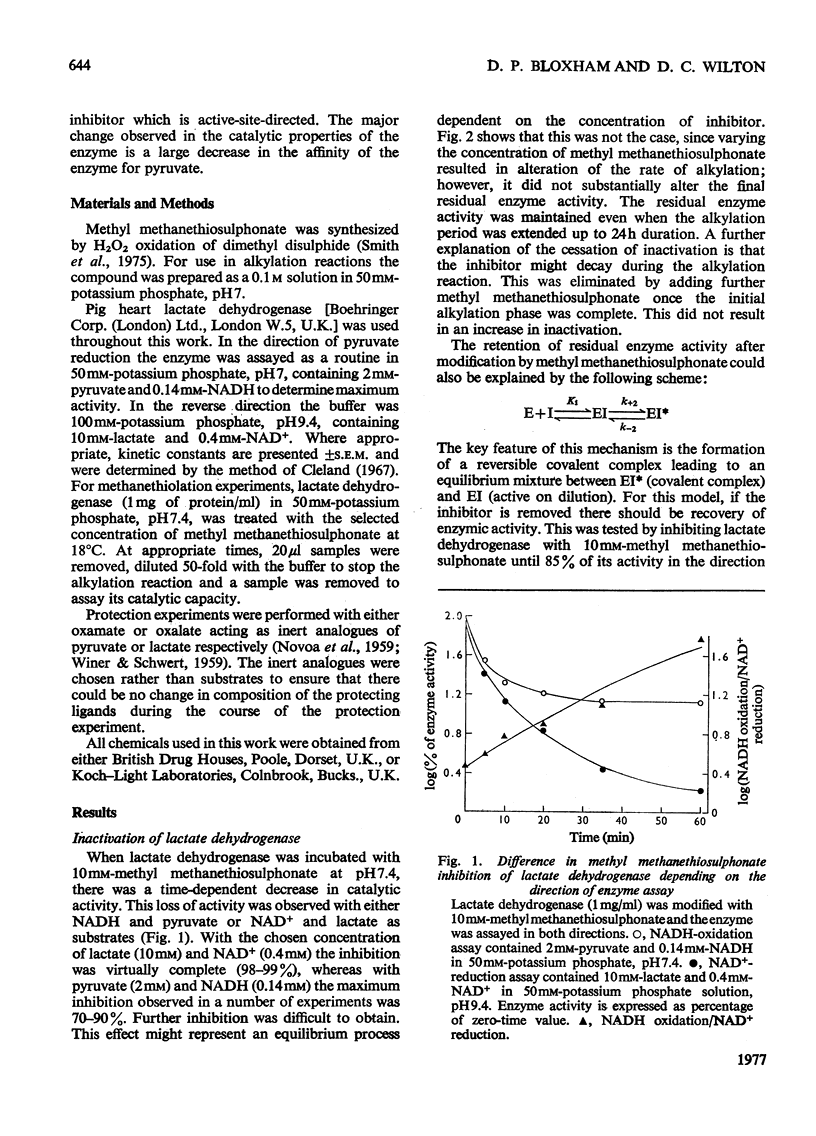
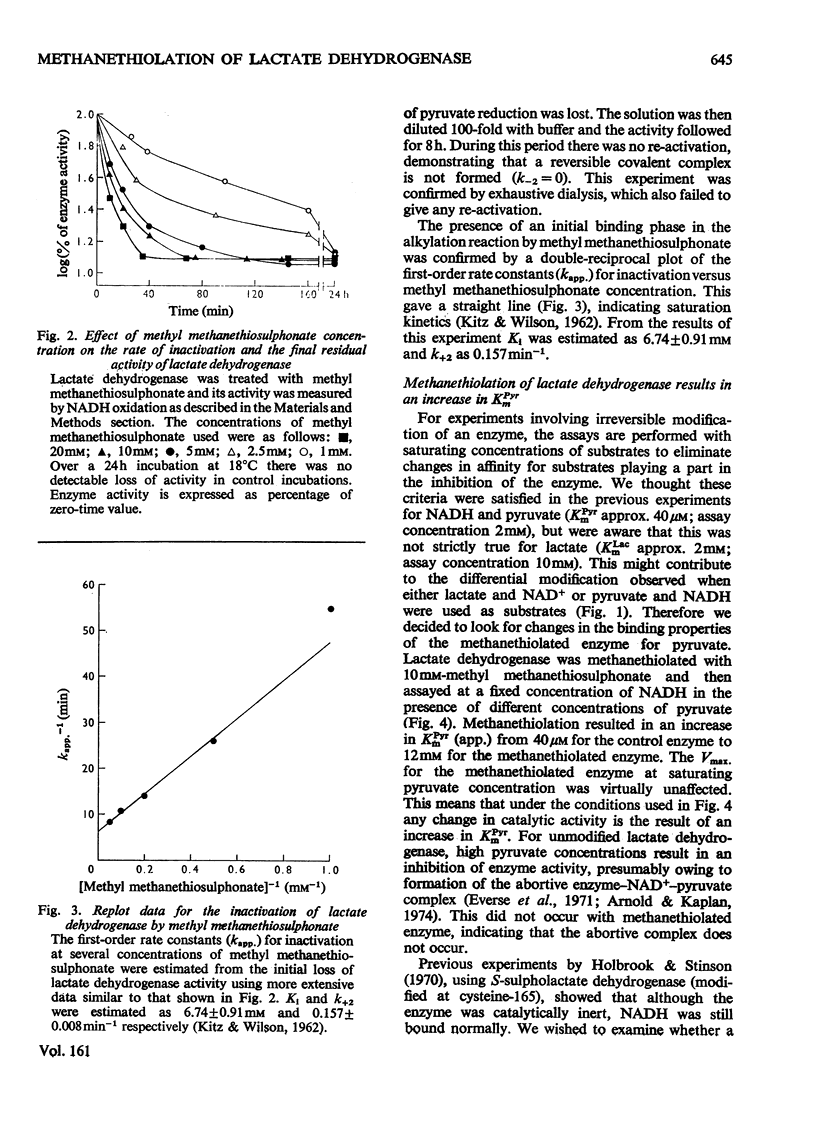
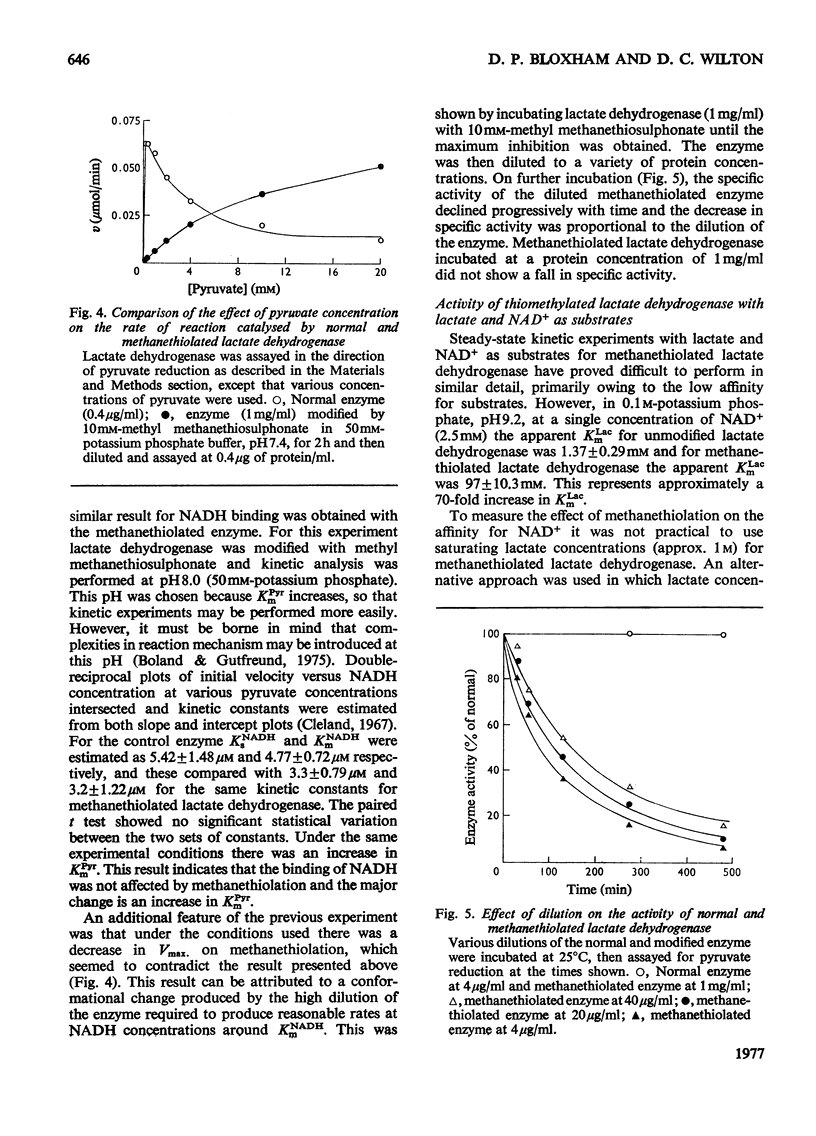
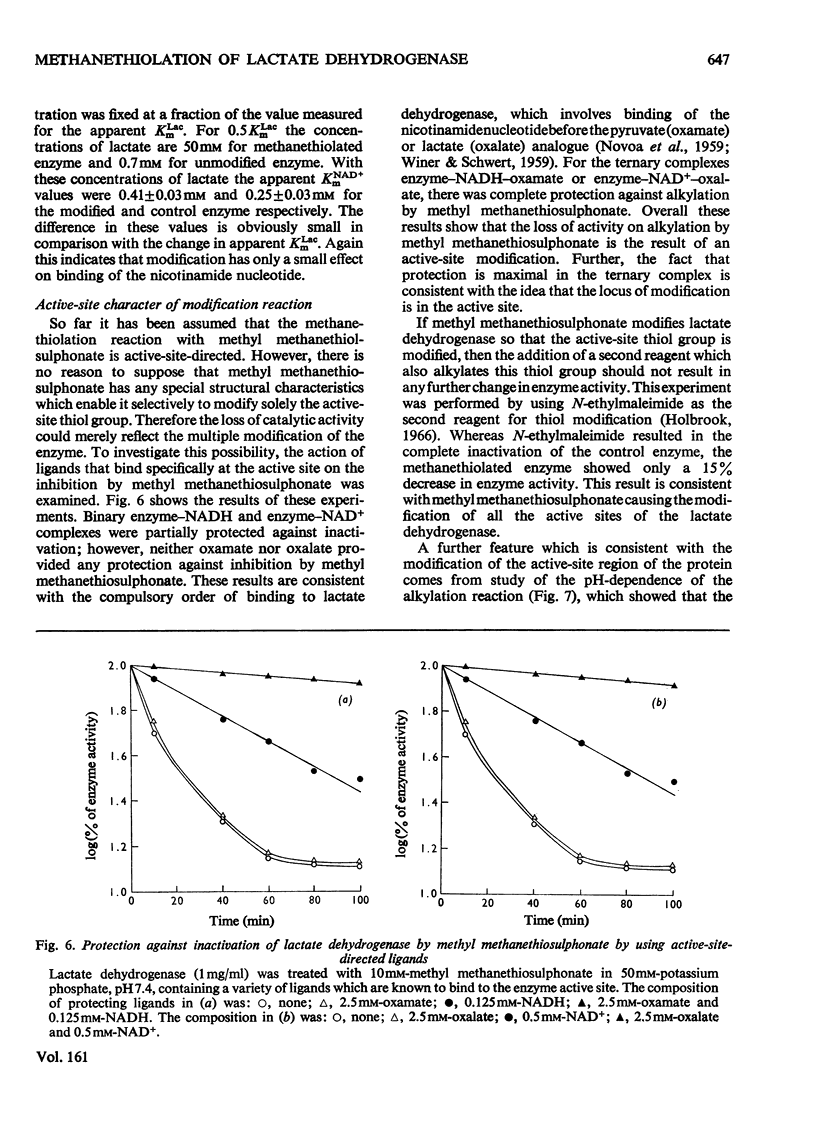
Methyl methanethiosulphonate was used to produce a modification of the essential thiol group in lactate dehydrogenase which leaves the enzyme catalytically active. Methyl methanethiosulphonate produced a progressive inhibition of enzyme activity, with 2mM-pyruvate and 0.14mM-NADH as substrates, which ceased once the enzyme had lost 70-90% of its activity. In contrast, with 10mM-lactate and 0.4mM-NAD+ as substrates the enzyme was virtually completely inhibited. The observed inhibition was critically dependent on the chosen substrate concentration, since methanethiolation with methyl methanethiosulphonate resulted in a large decrease in affinity for pyruvate. At 0.14mM-NADH, methanethiolation increased the apparent KmPyr from from 40micronM for the control enzyme to 12mM for the modified enzyme. Steady-state kinetics showed that there was not a statistically significant change in either KmNADH or KsNADH. At saturating NADH and pyruvate concentrations, the Vmax. was virtually unaffected for the methanethiolated enzyme. However, a decrease in Vmax. was observed when the modified enzyme was incubated in dilute solution. The modification of lactate dehydrogenase by methyl methanethiosulphonate involved the active site, since inhibition was completely prevented by substrate-analogue pairs such as NADH and oxamate or NAD+ and oxalate. The formation of complexes between methanethiolated lactate dehydrogenase and substrates or substrate analogues can also be shown by re-activation experiments. The methanethiolated enzyme was re-activated in a time-dependent reaction by dithiothreitol and this was prevented by oxamate, by NADH and by NADH plus oxamate in increasing order of effectiveness. The results of this work are interpreted in terms of a role for the essential thiol group in the binding of substrates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Ford G. C., Koekoek R., Lentz P. J., McPherson A., Jr, Rossmann M. G., Smiley I. E., Schevitz R. W., Wonacott A. J. Structure of lactate dehydrogenase at 2-8 A resolution. Nature. 1970 Sep 12;227(5263):1098–1103. doi: 10.1038/2271098a0. [DOI] [PubMed] [Google Scholar]
- Arnold L. J., Jr, Kaplan N. O. The structure of the abortive diphosphopyridine nucleotide-pyruvate-lactate dehydrogenase ternary complex as determined by proton magnetic resonance analysis. J Biol Chem. 1974 Jan 25;249(2):652–655. [PubMed] [Google Scholar]
- Bloxham D. P., Clark M. G., Holland P. C., Lardy H. A. Inactivation of phosphofructokinase by 6-mercapto-9- -D-ribofuranosylpurine 5'-triphosphate. Biochemistry. 1973 Apr 10;12(8):1596–1601. doi: 10.1021/bi00732a021. [DOI] [PubMed] [Google Scholar]
- Bloxham D. P., Giles I. G., Wilton D. C., Akhtar M. The mechanism of the bond forming events in pyridine nucleotide linked oxidoreductases. Studies with epoxide inhibitors of lactic dehydrogenase and beta-hydroxybutyrate dehydrogenase. Biochemistry. 1975 May 20;14(10):2235–2241. doi: 10.1021/bi00681a030. [DOI] [PubMed] [Google Scholar]
- Boland M. J., Gutfreund H. Pig heart lactate dehydrogenase. Binding of pyruvate and the interconversion of pyruvate-containing ternary complexes. Biochem J. 1975 Dec;151(3):715–727. doi: 10.1042/bj1510715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Everse J., Barnett R. E., Thorne C. J., Kaplan N. O. The formation of ternary complexes by diphosphopyridine nucleotide-dependent dehydrogenases. Arch Biochem Biophys. 1971 Apr;143(2):444–460. doi: 10.1016/0003-9861(71)90230-x. [DOI] [PubMed] [Google Scholar]
- Fondy T. P., Everse J., Driscoll G. A., Castillo F., Stolzenbach F. E., Kaplan N. O. The comparative enzymology of lactic dehydrogenases. IV. Function of sulfhydryl groups in lactic dehydrogenases and the sequence around the essential group. J Biol Chem. 1965 Nov;240(11):4219–4234. [PubMed] [Google Scholar]
- Holbrook J. J., Gutfreund H. Approaches to the study of enzyme mechanisms lactate dehydrogenase. FEBS Lett. 1973 Apr 15;31(2):157–169. doi: 10.1016/0014-5793(73)80095-x. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Pfleiderer G. Bedeutung von SH-Gruppen für die enzymatische Aktivität. 3. Eine Methode, um die essentiellen Cysteinreste in nativer Schweineherz-Lactatdehydrogenase (Isozym I) radioaktiv zu markieren. Biochem Z. 1965 Jun 3;342(1):111–114. [PubMed] [Google Scholar]
- Holbrook J. J., Stinson R. A. Reactivity of the essential thiol group of lactate dehydrogenase and substrate binding. Biochem J. 1970 Nov;120(2):289–297. doi: 10.1042/bj1200289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Stinson R. A. The use of ternary complexes to study ionizations and isomerizations during catalysis by lactate dehydrogenase. Biochem J. 1973 Apr;131(4):739–748. doi: 10.1042/bj1310739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J. The importance of SH-groups for enzymic activity. V. The coenzyme-binding capacity of pig heart lactate dehydrogenase, isozyme I, after inhibition by various maleinimides. Biochem Z. 1966 Mar 28;344(2):141–152. [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Kolb D. A., Weber G. Quantitative demonstration of the reciprocity of ligand effects in the ternary complex of chicken heart lactate dehydrogenase with nicotinamide adenine dinucleotide oxalate. Biochemistry. 1975 Oct 7;14(20):4471–4476. doi: 10.1021/bi00691a020. [DOI] [PubMed] [Google Scholar]
- NEILANDS J. B. Studies on lactic dehydrogenase of heart. III. Action of inhibitors. J Biol Chem. 1954 May;208(1):225–230. [PubMed] [Google Scholar]
- NOVOA W. B., WINER A. D., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. V. Inhibition by oxamate and by oxalate. J Biol Chem. 1959 May;234(5):1143–1148. [PubMed] [Google Scholar]
- Nishimura J. S., Kenyon G. L., Smith D. J. Reversible modification of the sulfhydryl groups of Escherichia coli succinic thiokinase with methanethiolating reagents, 5.5'-Dithio-bis(2-nitrobenzoic acid), p-hydroxymercuribenzoate, and ethylmercurithiosalicylate. Arch Biochem Biophys. 1975 Oct;170(2):461–467. doi: 10.1016/0003-9861(75)90141-1. [DOI] [PubMed] [Google Scholar]
- Schwert G. W., Miller B. R., Peanasky R. J. Lactic dehydrogenase. X. A re-evaluation of the effects of pH upon the kinetics of the reaction. J Biol Chem. 1967 Jul 25;242(14):3245–3252. [PubMed] [Google Scholar]
- Smiley I. E., Koekoek R., Adams M. J., Rossmann M. G. The 5 A resolution structure of an abortive ternary complex of lactate dehydrogenase and its comparison with the apo-enzyme. J Mol Biol. 1971 Feb 14;55(3):467–475. doi: 10.1016/0022-2836(71)90330-5. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Maggio E. T., Kenyon G. L. Simple alkanethiol groups for temporary blocking of sulfhydryl groups of enzymes. Biochemistry. 1975 Feb 25;14(4):766–771. doi: 10.1021/bi00675a019. [DOI] [PubMed] [Google Scholar]
- VERLICK S. F. Fluorescence spectra and polarization of glyceraldehyde-3-phosphate and lactic dehydrogenase coenzyme complexes. J Biol Chem. 1958 Dec;233(6):1455–1467. [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. VII. Fluorescence spectra of ternary complexes of lactic dehydrogenase, reduced diphosphopyridine nucleotide, and carboxylic acids. J Biol Chem. 1959 May;234(5):1155–1161. [PubMed] [Google Scholar]
- Whitaker J. R., Yates D. W., Bennett N. G., Holbrook J. J., Gutfreund H. The identification of intermediates in the reaction of pig heart lactate dehydrogenase with its substrates. Biochem J. 1974 Jun;139(3):677–697. doi: 10.1042/bj1390677. [DOI] [PMC free article] [PubMed] [Google Scholar]


