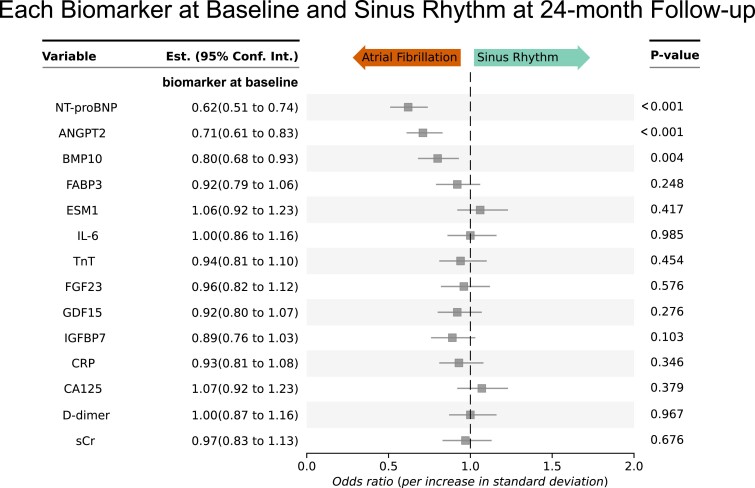
Figure 4.
Internal validation: angiopoietin 2, bone morphogenetic protein 10, and NT-proBNP biomarkers at baseline predict sinus rhythm at 24-month follow-up even after correction for multiple confounders. Odds ratios are shown for sinus rhythm at 24-month follow-up. This analysis provides an internal validation of the biomarkers predicting sinus rhythm at 12-month follow-up (Figure 1). All odds ratios are corrected for clinical age, sex, study site, rhythm at baseline, randomized treatment group (early rhythm control or usual care), body mass index, diastolic blood pressure, and left ventricular ejection fraction, those clinical features that were associated with outcomes including sinus rhythm in the main EAST-AFNET 4 trial.4 Low concentrations of NT-proBNP, ANGPT2, and BMP10 predict sinus rhythm at 24-month follow-up in patients. Accordingly, high concentrations predict lack of sinus rhythm at 24-month follow-up. ANGPT2, angiopoietin 2; BMP10, bone morphogenetic protein 10; CA125, cancer antigen 125; CRP, C-reactive protein; D-dimer, ESM1, endothelial specific molecule 1; FABP3, fatty acid binding protein 3; FGF23, fibroblast growth factor 23; GDF15, growth differentiation factor 15; IGFBP7, insulin-like growth factor binding protein 7; IL-6, interleukin-6; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TnT, cardiac troponin; sCr, serum creatinine