Figure 1.
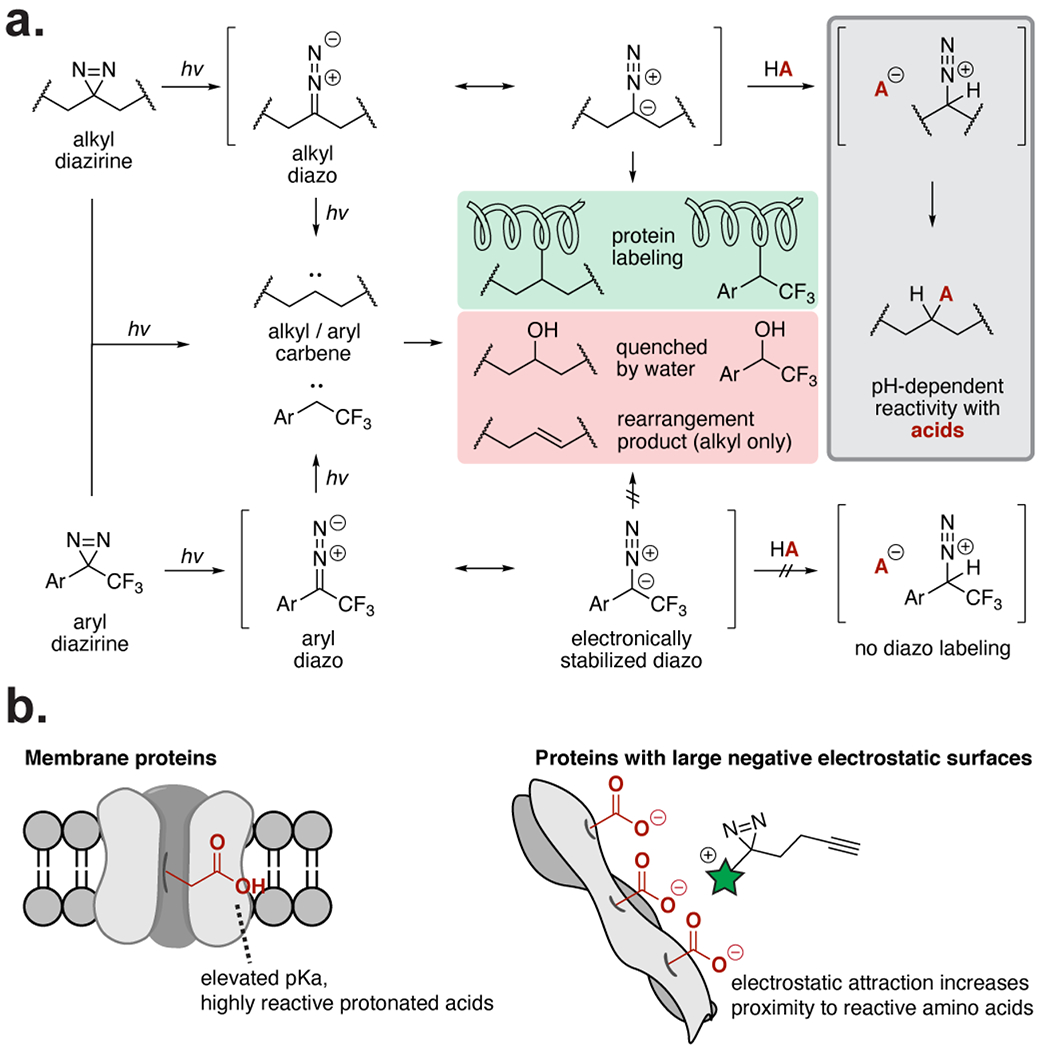
Overview of diazirine reactivity pathways. (a) Alkyl and aryl diazirines form carbene and diazo intermediates upon irradiation. Carbenes label nearby proteins, but are rapidly quenched if no protein substrate is nearby. Alkyl diazo intermediates react selectively with acids, while electronically stabilized aryl diazo intermediates do not. (b) The acid-selectivity of the alkyl diazo intermediates causes increased labeling of membrane proteins, which have more reactive protonated carboxylic acids, and proteins with large negative electrostatic surfaces. Labeling of these protein surfaces increases when the PAL probe (highlighted in green) is positively charged.
