Figure 2.
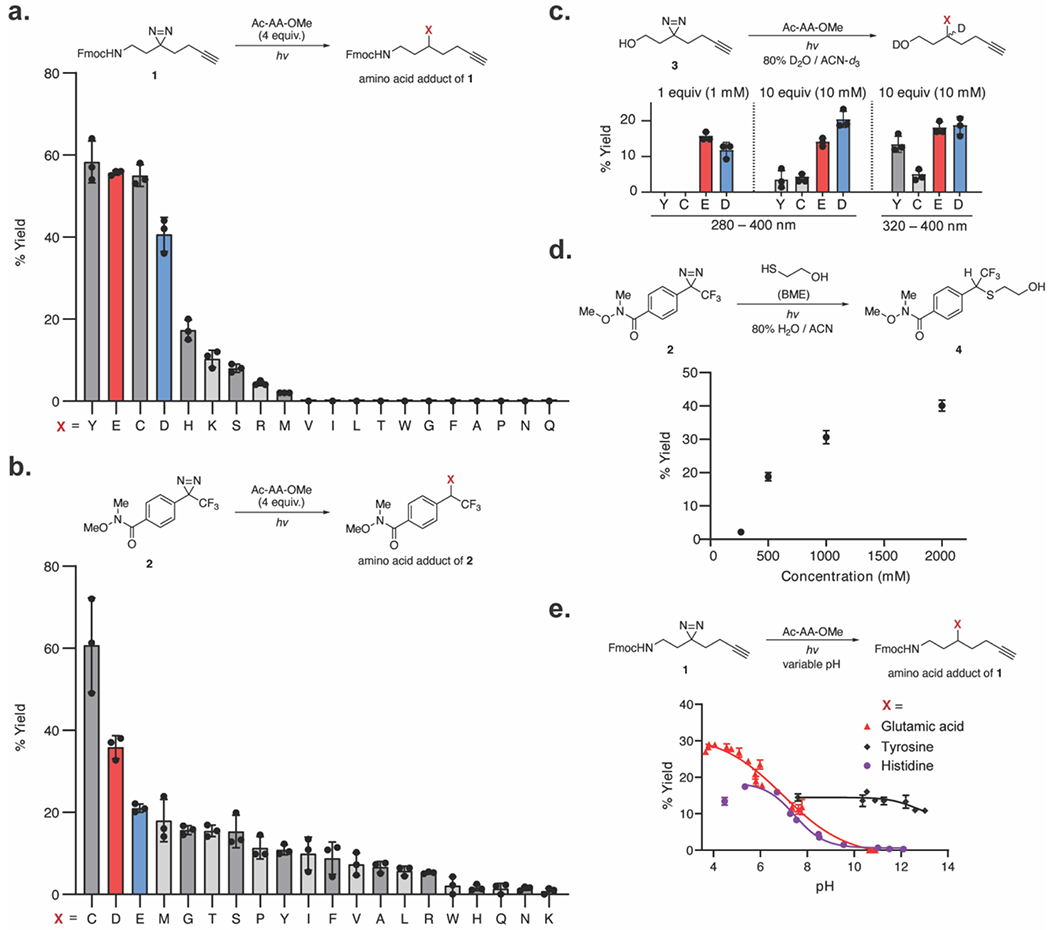
Reactivity of alkyl and aryl diazirines with the 20 natural amino acids. (a) Reactivity of alkyl diazirines with the 20 N-acetyl, O-Me protected amino acids (4 equiv) in neat conditions. Yields calculated against an internal standard by LC-MS. (b) Reactivity of aryl diazirines with the 20 amino acids (4 equiv) in neat conditions. Yields calculated against an internal standard by [isp]19F NMR. (c) Reactivity of alkyl diazirines with the 20 amino acids in solution (1 mM 3, 1 mM or 10 mM amino acid) with 280–400 nm or 320–400 nm excitation. Only reactive amino acids are displayed. Yields calculated against an internal standard by 1H NMR. (d) Reaction of aryl diazirine 2 with β-mercapto ethanol at varying concentrations in aqueous conditions. Yields calculated with relative integration by LC-MS. (e) Yield of alkyl diazirines with individual amino acids as a function of pH. Yields calculated against an internal standard by LC-MS. Plotted values show the mean with error bars representing the standard deviation with n = 3.
