Abstract
Oat-based liquid and semi-solid dairy alternatives require extractable proteins for nutritional and technological purposes. However, oats are industrially heat treated (‘kilned’) to inactivate endogenous lipases thereby avoiding rancidity development. Such heat treatment results in a protein extractability decrease. We here investigated the possibility of directing oat groat heat treatment conditions [oat groat moisture content (13.0–20.0%), heating temperature (80–100 °C) and heating time (15–45 min)] on a lab-scale to achieve complete enzyme inactivation, with peroxidase activity as a marker, while maintaining high protein extractability. Non-heat-treated and industrially heat-treated oats were included as reference samples. The peroxidase activity and protein extractability of lab-scale heat-treated oats decreased with an increase in moisture content, heating temperature and time. Several lab-scale heat-treated oats for which complete peroxidase inactivation was observed, had significantly higher protein extractabilities (31–59%) than industrially kilned oats (21%). The activity of endogenous lipases was determined for a selected sample set. Lipases required milder heat treatment conditions for complete inactivation than peroxidases. Such milder heat treatment led to samples with protein extractabilities between 31 and 65%. A notable observation was that heat treating oats (≥90 °C) caused clumping of the intracellular material of the aleurone cells, likely due to protein aggregation. The main conclusion of this study is that oat heat treatment conditions can be altered successfully to achieve complete enzyme inactivation while maintaining high protein extractability. The obtained insights could lead to the development of oat-based products with higher protein content and desired shelf stability.
Keywords: Oats, Kilning, Peroxidase activity, Lipase activity, Proteins
Graphical abstract
Highlights
-
•
Industrial heat treatment of oats results in reduced protein extractability.
-
•
Oat groat heat treatment conditions can be directed to retain high protein extractability.
-
•
Inactivation of lipases requires less severe heat treatment than that of peroxidases.
-
•
Clumping of oat aleurone intracellular material occurred upon heat treating oats.
1. Introduction
To ensure sustainable food production and security in the coming decades, a partial transition from animal-based to plant-based diets is necessary (Aiking and de Boer, 2020). Proteins are an essential functional component in the production of many plant-based foods (Sim et al., 2021), including alternatives to milk (McClements et al., 2019; McClements and Grossmann, 2021) and yogurt (Montemurro et al., 2021; Zang et al., 2024). Oats (Avena sativa L.) are particularly interesting for these applications due to their well-balanced amino acid composition and gluten-free nature (Klose and Arendt, 2012; Mäkinen et al., 2017; Welch, 2011). Oats also have a high protein content (12–15%) (Sunilkumar et al., 2017), the majority of which are albumins (1–12%) and globulins (70–80%) (Klose and Arendt, 2012; Lásztity, 1998; Mäkinen et al., 2017). The main oat protein is the 12S globulin, a hexamer (320 kDa) composed of monomers containing an acidic α-subunit (32 kDa) and a basic β-subunit (22 kDa) linked by a disulfide bond (Klose and Arendt, 2012). Besides protein, oats are a source of avenanthramides (antioxidants unique to oats) and the dietary fibers β-D-glucan and arabinoxylan (Welch, 2011).
The production of many dairy alternatives starts from a liquid base, which is in essence a water extract of a plant-based raw material, in some cases aided by enzymatic treatments (Aydar et al., 2020). Most commercially available oat-based drinks and yogurt alternatives have very low protein content, indicating that only a fraction of oat proteins ends up in the liquid bases used to produce these products (Spaen and Silva, 2021). This limited protein extractability is due to (i) the physical entrapment of proteins within cellular structures of the oat groat (Janssen et al., 2023; Miller and Fulcher, 2011) and (ii) protein structural changes caused by the heat treatment (‘kilning’) applied to oat groats to inactivate lipases, preventing lipid hydrolysis and rancidity development (Girardet and Webster, 2011; Pålsson et al., 2024). Runyon et al. (2015) observed a decrease in oat protein extractability from 75% to 36% (at pH 9.5) after heat treating oat groats. Similarly, Janssen et al. (2023) reported a protein extractability of only 23% (at pH 9.0) for defatted whole meal from industrially heat-treated oats. Until recently, industrially heat-treated oats were mostly further processed into flakes or whole meal for solid food applications like muesli and ready-to-eat cereals (Girardet and Webster, 2011), where reduced protein extractability is not a concern. However, for liquid and semi-solid dairy alternatives, extractable protein is desired and severe heat treatment is ill-suited. In industry, the oat groat heat treatment is typically carried out in a continuous radiator system comprised of three steps: (i) steam injection to increase groat temperature (∼80 °C) and moisture content (16–18%), (ii) isothermal heat treatment (100–120 °C), and (iii) simultaneous cooling (21–25 °C) and drying. The entire process typically lasts 90–120 min (Ganẞmann and Vorwerck, 1995; Girardet and Webster, 2011).
Oat lipase inactivation depends on the temperature, time and groat moisture content during heat treatment (Ekstrand et al., 1992; Ganẞmann and Vorwerck, 1995; Hutchinson et al., 1951). For instance, oats with 30% moisture heat treated at 80 °C until a moisture content of 16% was reached, had 3.3 times lower lipase activity than those treated at 60 °C (Ekstrand et al., 1992). Lehtinen et al. (2003) found that lipid hydrolysis (assessed by analyzing the lipid composition) was more pronounced in mildly heat-treated oats (17% moisture, 90 °C, 20 min) than in intensively heat-treated oats (20% moisture, 100 °C, 40 min). Elsewhere, heat treating oats at a moisture content of 20% and 64 °C (60 min) resulted in the same degree of lipase inactivation as oats heat treated at 6% moisture and 120 °C (60 min) (Hutchinson et al., 1951). Finally, lipase inactivation has been observed to occur more rapidly when heat treating (80 °C) oat groats with 20% moisture than with 14% moisture (Ganẞmann and Vorwerck, 1995). Although lipases play a critical role in the development of rancidity (Decker et al., 2014; Lehtinen and Laakso, 2004), in the industry, the peroxidase activity is used as a robust measure for oat groat shelf stability because peroxidases are considered the most heat-stable enzymes in the oat groat (Ekstrand et al., 1992; Ganẞmann and Vorwerck, 1995). This implies that it may well be possible to inactivate oat lipases under milder heat treatment conditions than those required for completely inactivating peroxidases. At the same time, it is here hypothesized that thermal enzyme inactivation, particularly lipases, is possible while minimizing the impact on the main oat storage proteins, the 12S globulins. Indeed, oat 12S globulins have a higher denaturation temperature (110 °C) than oat albumins, including lipases (Klose and Arendt, 2012; Ma and Harwalkar, 1987; Mäkinen et al., 2017). However, to the best of our knowledge, the impact of varying heat treatment conditions on the protein extractability of oats has not yet been systematically investigated in literature.
Successfully achieving complete enzyme inactivation while maintaining high protein extractability would be highly beneficial for producers of oat-based dairy alternatives, potentially leading to products with higher protein content and desired shelf stability. The aim of this study is to direct the heat treatment conditions for oat groats to retain high oat protein extractability. To achieve this, non-heat-treated oat groats were heat treated at different temperatures (80–100 °C), times (15–45 min) and groat moisture contents (13.0–20.0%). The peroxidase activities (as a proxy for overall enzyme activity), lipase activities (for selected samples) and protein extractabilities of oat whole meal prepared from these lab-scale heat-treated oat groats were measured and compared with those of non-heat-treated and industrially heat-treated oat groats. Insight into the impact of various heat treatment conditions on the oat groat microstructure was gained by microscopically visualizing embedded oat groat cross sections.
2. Materials and methods
2.1. Materials
Non-heat-treated and industrially heat-treated oat groats from the same batch, comprising a mixture of different cultivars harvested in 2020 in Finland, were kindly donated by Maselis N.V. (Roeselare, Belgium). The industrial heat treatment conditions applied to these oat groats were not disclosed to the authors, but it is known that a peroxidase activity of zero is targeted. The oat groats were stored at −20 °C until further use.
Hexane and hydrogen peroxide (H2O2) (30% w/v) were purchased from Chem-Lab Analytical (Zedelgem, Belgium). Sodium hydroxide (NaOH) (pellets, purity 98.5%), sodium carbonate (Na2CO3) (purity 99.5%), and dimethylsulfoxide (DMSO, purity 99.8%) and bovine serum albumin (BSA) were obtained from Acros Organics (The Hague, Netherlands). Formaldehyde (37% w/v) and absolute ethanol (analytical grade) were from Thermo Fisher Scientific (Aalst, Belgium). Potassium hydroxide (KOH), sodium dodecyl sulfate (SDS), sodium dihydrogen phosphate dihydrate (NaH2PO4.2H2O), potassium sodium (L+) tartrate tetrahydrate (NaK-tartrate.4H2O) and copper(II)sulfate pentahydrate (CuSO4.5H2O) were from VWR International (Leuven, Belgium). Naphthol Blue Black, potassium phosphate monobasic (KH2PO4), sodium phosphate dibasic dihydrate (Na2HPO4.2H2O), sodium phosphate dibasic dodecahydrate (Na2HPO4.12H2O), glutaraldehyde solution (25% v/v), Triton X-100, ethanol (99% v/v) denatured, guaiacol, Folin-Ciocalteu's phenol reagent and 4-nitrophenyl butyrate were obtained from Sigma-Aldrich (Bornem, Belgium). The HistoResin Embedding Kit was purchased from Leica Biosystems (Leica Biosystems Nussloch GmbH, Nussloch, Germany).
2.2. Heat treatment of oat groats
A lab-scale oat groat heat treatment procedure was developed and involved three subsequent steps: (i) tempering, (ii) isothermal heat treatment using an autoclave, and (iii) drying of the oat groats. A detailed overview of the different combinations of heat treatment conditions is provided in Table S1 in the supplementary information. A general overview of the experimental set-up of this study is shown in Fig. 1.
Fig. 1.
Schematic overview of the experimental set-up.
2.2.1. Tempering of oat groats
Non-heat-treated oat groats had an initial moisture content of 13.0%, as determined with AACCI method 44–19.01 (AACCI, 1999), with the drying time extended to 16 h. To reach moisture contents of 16.5% or 20.0%, deionized water was added and the oat groats were stored at 4 °C for 8 h and shaken every 2 h. Subsequently, the oat groats were equilibrated at room temperature for 16 h to ensure uniform water distribution. After tempering, the moisture content of the oat groats was verified to be as intended.
2.2.2. Heat treatment of oat groats
The oat groats prepared in section 2.2.1 were subjected to an isothermal heat treatment using a Systec VX55 autoclave (Analis, Namur, Belgium). Oat groats (40 g, with moisture contents of 13.0, 16.5 or 20.0%) were spread out as a single layer (to allow rapid and uniform heat transfer) at the bottom of horizontally positioned 2.0 L Schott bottles in the autoclave. These samples were then subjected to treatments comprised of varying combinations of temperature (80, 90 and 100 °C) and time (15, 30 and 45 min) (Table S1). The heating time refers to the holding phase in the autoclave, excluding the warm-up phase. The total heat load (°C.min) of the heat treatments, including both the warm up and holding phases, was determined by measuring the temperature inside the Schott bottle over time using temperature probes (Ellab TrackSense Pro Wireless Data Logger, Hengelo, The Netherlands), and was here defined as the area under the measured temperature-time curves (Table S1).
2.2.3. Drying of oat groats
Immediately following the heat treatment, the differently heat-treated oat groats were transferred onto stainless steel sieves, dried in a hot air oven at 50 °C for 2 h, pooled and stored at −20 °C until further use.
2.3. Light microscopy imaging of oat groats
Non-heat-treated, industrially heat-treated and a selected set of lab-scale heat-treated oat groats were embedded in Historesin as described by Dornez et al. (2011a) (Fig. 1). The oat groats were moistened between wetted filter paper for 16 h at 4 °C, after which the ends of the groats were removed using a scalpel. The groats were then fixed with 3.0% w/v paraformaldehyde and 1.0% v/v glutaraldehyde in 0.1 M sodium potassium phosphate buffer (pH 7.0), washed with deionized water (three times for 30 min each), dehydrated using a graded ethanol series (50% v/v, 70% v/v, 95% v/v twice for 60 min each, and 100% v/v for 16 h), and infiltrated with the Historesin Embedding Kit (Leica Biosystems Nussloch GmbH). Transverse sections (4 μm) of the groats were obtained with a Leica RM2255 rotary microtome (Leica Biosystems Nussloch GmbH) and transferred to microscopy slides. The sections were stained with Naphthol Blue Black (1.0% w/v in deionized water) for 4 min to visualize proteins (Hermans et al., 2021), rinsed with deionized water and air-dried. The stained sections were then analyzed using a Nikon Eclipse 80i epifluorescence microscope (Nikon Inc., New York, USA) and bright-field images were captured at 200x magnification.
2.4. Preparation of defatted oat whole meal samples
Non-heat-treated, industrially heat-treated and lab-scale heat-treated oat groats were milled into oat whole meal (OW) using a Cyclotec Sample mill 1093 (Foss, Högenäs, Sweden) equipped with a 500 μm sieve (Fig. 1). The OW was stored at −20 °C until a defatting step, which was executed within 7 days of the milling step to limit the development of rancidity thereby avoiding its potential interference with methods for determining enzymatic activity (2.6, 2.7) and protein extractability (section 2.8). OW was defatted by suspending it in hexane (1:5 w/v ratio). The OW-hexane suspension was continuously shaken (1 h, 150 rpm, room temperature) and subsequently centrifuged (10 min, 2,000 g, room temperature). The supernatant, containing the lipid fraction, was discarded by decanting. The extraction procedure was repeated and afterwards, the obtained defatted oat whole meal (DOW) was dried overnight and spread on filter paper to allow evaporation of residual hexane. The DOW was ground using a mortar and stored at 4 °C until further use. DOW obtained from non-heat-treated, industrially heat-treated and lab-scale heat-treated oats will be referred to as DOWNHT, DOWIHT and DOWLHT, respectively. For DOWLHT, a sample code indicating the oat groat moisture content (in superscript), heating temperature (first number in subscript) and heating time (second number in subscript) will be used: .
2.5. Moisture and protein content of defatted oat whole meal samples
The moisture contents of DOWNHT, DOWIHT and DOWLHT samples were determined according to AACCI method 44–19.01 (AACCI, 1999). The protein contents of DOWNHT, DOWIHT and DOWLHT samples were determined with an Elemental Analyzer 1108 (Carlo Erba Instruments, Milan, Italy) using a nitrogen-to-protein conversion factor of 5.8 (Mariotti et al., 2008) (Fig. 1). All analyses were done in triplicate.
2.6. Peroxidase activity of defatted oat whole meal samples
The peroxidase activities of DOWNHT, DOWIHT and DOWLHT samples were measured based on the procedure described by Pütter (1974), with minor adjustments (Fig. 1). In short, 1.0 g of DOW was suspended in 10.0 mL potassium phosphate buffer (0.1 M, pH 5.0). After centrifugation (10 min, 1250 g), the colorimetric reaction was initiated by mixing the supernatant (0.1 mL) with potassium phosphate buffer (2.8 mL), guaiacol (0.05 mL, 0.018 M) and hydrogen peroxide (0.05 mL, 0.03% w/v) as substrates. After exactly 1.5 min of incubation at room temperature, the absorbance was measured at 436 nm every min for 7 min. The signal of the blank, containing only the supernatant (0.1 mL) and potassium phosphate buffer (2.9 mL), was subtracted from the sample readings. The rate at which the absorbance increased over time was used to calculate the peroxidase activity, expressed as units per g dry matter (dm) of sample. One unit corresponds to the enzyme activity that increased the absorbance at 436 nm by 1.0 per min of incubation. Analyses were done in triplicate.
2.7. Lipase activity of defatted oat whole meal samples
The method for determining the lipase activities of DOWNHT, DOWIHT and a selected set of DOWLHT samples was adapted from Yang et al. (2017) and Wei et al. (2020), using p-nitrophenyl butyrate as a substrate (Fig. 1). Analyses were done in triplicate. A fresh stock solution of 200 mM p-nitrophenyl butyrate in pure DMSO (99.8%) was prepared daily for use. The p-nitrophenyl butyrate working solution was prepared by diluting 0.1 mL of the stock solution into 4.9 mL of sodium phosphate buffer (0.05 M, pH 8.0) containing 0.1% (w/v) Triton X-100, and was used immediately. A DMSO blank solution, prepared by diluting 0.1 mL pure DMSO into 4.9 mL sodium phosphate buffer (0.05 M, pH 8.0) containing 0.1% (w/v) Triton X-100, was also prepared daily. About 0.5 g of DOW was accurately weighed and suspended in 5.0 mL of sodium phosphate buffer (0.05 M, pH 8.0) containing 0.1% (w/v) Triton X-100. After centrifugation (10 min, 10,000 g, room temperature) and filtration (Millex-HP, 0.45 μm, polyethersulfone; Millipore, Carrigtwohill, Ireland) of the supernatant, the colorimetric reaction was initiated by mixing 0.25 mL of the supernatant with 0.5 mL of the p-nitrophenyl butyrate working solution and 2.0 mL of sodium phosphate buffer (0.05 M, pH 8.0) containing 0.1% (w/v) Triton X-100. After exactly 5 min of incubation at room temperature, the absorbance was measured at 405 nm against a blank of sodium phosphate buffer (0.05 M, pH 8.0) containing 0.1% (w/v) Triton X-100. A sample blank [0.25 mL supernatant, 0.5 mL DMSO blank solution and 2.0 mL sodium phosphate buffer (0.05 M, pH 8.0) containing 0.1% (w/v) Triton X-100] and a p-nitrophenyl butyrate blank [0.5 mL p-nitrophenyl butyrate working solution and 2.25 mL sodium phosphate buffer (0.05 M, pH 8.0 containing 0.1% (w/v) Triton X-100] were also included. The lipase activity is expressed as units per g dm of DOW, where one unit corresponds to the enzyme activity that increases the absorbance at 405 nm by 1.0 per min of incubation, and was calculated as follows:
with V the volume of sodium phosphate buffer used (mL), m the sample weight (g dm) and t the incubation time (min).
2.8. Protein extractability of defatted oat whole meal samples
DOWNHT, DOWIHT and DOWLHT samples were suspended in deionized water (1:10 w/v ratio). The pH of the suspension was adjusted to 9.0 using small amounts of 1.0 M NaOH. The suspension was shaken (30 min, 175 rpm, room temperature) and centrifuged (10 min, 10,000 g, room temperature). The obtained supernatant was collected and the extraction was repeated on the pellet. Both supernatants were pooled and weighed to allow calculating protein extractabilities. Extracts were prepared in triplicate.
The protein concentrations of DOWNHT, DOWIHT and DOWLHT extracts were determined (Fig. 1) by diluting each extract 1:1 (v:v) with 0.1 M sodium phosphate buffer containing 2.0% (w/v) SDS. After shaking (60 min, 150 rpm, room temperature), the mixture was further diluted 10 times in 0.05 M sodium phosphate buffer containing 1.0% (w/v) SDS. The protein concentration was determined with the Lowry method using bovine serum albumin as a standard (Lowry et al., 1951). Protein concentration analyses were done in triplicate for each of three independently prepared extracts. The protein extractability (%) was calculated as the relative amount of protein recovered from the starting material in the extract:
2.9. Statistical analysis
Statistical analysis was performed using JMP Pro 17 software (SAS Institute, Cary, North Carolina, USA). A one-way analysis of variance (P < 0.05) with Tukey multiple comparison procedure was performed to identify significant differences between mean values.
3. Results and discussion
Optimizing oat groat heat treatment conditions, i.e. conditions for which complete enzyme inactivation and high protein extractability are observed, is of great industrial relevance, as this can result in the production of oat-based dairy alternatives with higher protein content and desired shelf stability. To achieve this, the impact of oat groat moisture content (13.0–20.0%), heating temperature (80–100 °C) and heating time (15–45 min) on the peroxidase activities (an important industrial indicator for oat groat shelf stability) and protein extractabilities of DOW, as well as on the lipase activities of a selected sample set, were investigated.
3.1. The impact of varying oat groat heat treatment conditions on the peroxidase activities of defatted oat whole meals
Peroxidases are the most heat-stable enzymes in oats, which is why the inactivation of peroxidases is used as an indicator for oat groat shelf stability in the industry (Ganẞmann and Vorwerck, 1995). The peroxidase activity of DOWNHT was found to be 6.92 U/g dm, while, as expected, no peroxidase activity was detected for DOWIHT. Table 1 compares the peroxidase activities of DOWNHT, DOWIHT and all DOWLHT samples. The impact of oat groat moisture content, heating temperature and heating time at otherwise fixed sets of parameters on the peroxidase activity of DOW are visualized in Fig. S1 in the supplementary information.
Table 1.
The peroxidase activity (in U/g dm) and protein extractability (in %) of defatted oat whole meal (DOW) obtained from non-heat-treated, industrially heat-treated and lab-scale heat-treated oat groats. Abbreviations: DOWNHT = DOW from non-heat-treated oat groats, DOWIHT = DOW from industrially heat-treated oat groats, DOWLHT = DOW from lab-scale heat-treated oat groats, n.d. = not detected (below detection limit). The relative decrease in peroxidase activity (%) and protein extractability (%) compared to DOWNHT is shown as well. Values with a different capital letter within the same column are significantly different (α = 0.05). Standard deviations are depicted for the mean of triplicate measurements.
| Sample |
Peroxidase activity (U/g dm) | Peroxidase activity reduction (%) | Protein extractability (%) | Protein extractability reduction (%) | |||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Moisture content (%) | |||||
| DOWNHT | 6.92 ± 0.08 (BC) | – | 85.7 ± 2.4 (A) | – | |||
| DOWIHT | n.d. (K) | 100 | 20.6 ± 1.9 (Q) | 76 | |||
| DOWLHT | 80 | 15 | 13.0 | 6.65 ± 0.20 (CD) | 4 | 75.6 ± 2.5 (BCD) | 12 |
| 16.5 | 7.25 ± 0.20 (AB) | 0 | 81.0 ± 5.2 (AB) | 5 | |||
| 20.0 | 5.34 ± 0.20 (E) | 23 | 76.7 ± 4.4 (BC) | 10 | |||
| 30 | 13.0 | 7.50 ± 0.32 (A) | 0 | 84.5 ± 4.3 (A) | 1 | ||
| 16.5 | 4.66 ± 0.15 (F) | 33 | 75.8 ± 1.9 (BCD) | 11 | |||
| 20.0 | 4.08 ± 0.63 (G) | 41 | 68.5 ± 4.1 (EFG) | 20 | |||
| 45 | 13.0 | 6.44 ± 0.11 (CD) | 7 | 79.9 ± 4.8 (AB) | 7 | ||
| 16.5 | 5.17 ± 0.34 (EF) | 25 |
70.5 ± 2.1 (DEF) | 18 | |||
| 20.0 | 2.66 ± 0.13 (H) | 62 | 64.8 ± 4.7 (FGH) | 24 | |||
| 90 | 15 | 13.0 | 6.17 ± 0.18 (D) | 11 | 71.2 ± 2.4 (CDE) | 17 | |
| 16.5 | 2.98 ± 0.03 (H) | 57 | 71.3 ± 3.2 (CDE) | 17 | |||
| 20.0 | 0.70 ± 0.06 (IJ) | 90 | 60.2 ± 2.7 (H) | 30 | |||
| 30 | 13.0 | 4.92 ± 0.38 (EF) | 29 | 77.0 ± 2.9 (BC) | 10 | ||
| 16.5 | 1.13 ± 0.08 (I) | 84 | 66.5 ± 3.5 (EFG) | 22 | |||
| 20.0 | n.d. (K) | 100 | 53.6 ± 2.7 (IJ) | 37 | |||
| 45 | 13.0 | 3.79 ± 0.03 (G) | 45 | 63.7 ± 2.7 (GH) | 26 | ||
| 16.5 | 0.26 ± 0.15 (J) | 96 | 52.2 ± 4.2 (JK) | 39 | |||
| 20.0 | n.d. (K) | 100 | 46.3 ± 2.4 (L) | 46 | |||
| 100 | 15 | 13.0 | n.d. (K) | 100 | 59.0 ± 1.6 (HI) | 31 | |
| 16.5 | n.d. (K) | 100 | 41.9 ± 2.7 (LM) | 51 | |||
| 20.0 | n.d. (K) | 100 | 40.0 ± 3.9 (MN) | 53 | |||
| 30 | 13.0 | n.d. (K) | 100 | 46.4 ± 2.0 (KL) | 46 | ||
| 16.5 | n.d. (K) | 100 | 35.5 ± 3.7 (NO) | 59 | |||
| 20.0 | n.d. (K) | 100 | 31.4 ± 3.5 (OP) | 63 | |||
| 45 | 13.0 | n.d. (K) | 100 | 43.1 ± 2.4 (LM) | 50 | ||
| 16.5 | n.d. (K) | 100 | 32.0 ± 1.7 (OP) | 63 | |||
| 20.0 | n.d. (K) | 100 | 30.8 ± 2.3 (P) | 64 | |||
Fig. S1A shows the impact of varying oat groat moisture contents (13.0, 16.5 and 20.0%) during heat treatment at 90 °C for 30 min on the peroxidase activity of DOW. Peroxidase activities of 4.92 and 1.13 U/g dm were obtained for and , respectively, while for , no peroxidase activity was observed. This corresponds to reductions in peroxidase activity by 29%, 84% and 100%, respectively, compared to DOWNHT. A similar decreasing trend in peroxidase activity with increasing oat groat moisture content was observed for most heating durations at 80 or 90 °C (Table 1). For oat groats heat treated at 100 °C, no residual peroxidase activities were detected, regardless of the moisture content or heat treatment time (Table 1). In previous research by Gates et al. (2008), it was also noted that higher oat groat moisture content during heat treatment leads to more extensive peroxidase inactivation. An increased oat groat moisture content during heat treatment likely facilitates heat transfer throughout the oat groat and protein denaturation (Clark et al., 2020; Ekstrand et al., 1992), thereby enhancing peroxidase inactivation.
Fig. S1B illustrates the impact of heating temperature (80, 90 and 100 °C) on the peroxidase activities of DOW obtained from oat groats with a moisture content of 16.5% heat treated for 30 min. The peroxidase activity decreased significantly (P < 0.05) with increasing temperature. Indeed, for and , peroxidase activities of 4.66 and 1.13 U/g dm (corresponding to reductions in activity of 33 and 84%, respectively, compared to DOWNHT) were obtained, while for , complete peroxidase inactivation was achieved. This trend of decreasing peroxidase activity with increasing temperature was observed for most combinations of oat groat moisture contents and heating times (Table 1). Notably, at high oat groat moisture content (20.0%) and longer heating times (30 or 45 min), complete peroxidase inactivation occurred already at 90 °C (Table 1). In contrast, at 80 °C, complete peroxidase inactivation was not achieved at any of the moisture contents and heating times (Table 1).
Fig. S1C shows the impact of varying heating times (15, 30 and 45 min) on the peroxidase activities of DOW obtained from oat groats with a moisture content of 16.5% heat treated at 90 °C. A decline in peroxidase activity (P < 0.05) with increasing heating time was observed. Compared to DOWNHT, the peroxidase activity was reduced by 57%, 84% and 96% for , and , respectively. A similar decline in peroxidase activity was observed for , , and samples (Table 1). For , a heating time of 30 min was already sufficient to inactivate peroxidases completely. No significant (P > 0.05) impact of heating time on the peroxidase activity was observed for . For DOW obtained from oats heat treated at 100 °C, a heating time of 15 min was already sufficient to completely inactivate peroxidases, regardless of the moisture content of the groats (Table 1).
3.2. The impact of varying oat groat heat treatment conditions on the protein extractability of defatted oat whole meals
Table 1 shows the protein extractabilities of DOWNHT, DOWIHT and all DOWLHT samples, while the impact of oat groat moisture content, heating temperature and heating time at otherwise fixed sets of parameters on the protein extractability of DOW is visualized in Fig. S2. Protein extractability was calculated as the amount of protein in the extract relative to that in the amount of the corresponding DOW sample used for extraction (see section 2.8). The protein extractability of DOWNHT (85.6%) was four times higher (P < 0.05) than that of DOWIHT (20.6%). This shows that industrial oat heat treatment indeed significantly reduces the protein extractability, and agrees well with the study of Runyon et al. (2015), who reported a protein extractability decrease from 75% to 36% (at pH 9.5) upon heat treating oats. It should be noted that the protein extractability of DOWIHT might well vary in case heat-treated oat groats sourced from different suppliers would be used. It is likely, however, that most industrial oat groat heat treatments are relatively harsh and that they generally would lead to low protein extractabilities overall. In addition, the above mentioned protein extractabilities were recorded for DOW samples. It is possible that the defatting step influences the measured absolute protein extractability values. Nevertheless, since all OW samples were defatted, the obtained protein extractabilities remain comparable in relative terms.
Fig. S2A illustrates the impact of varying oat groat moisture content (13.0, 16.5 and 20.0%) during heat treatment at 90 °C for 30 min on the protein extractability of DOW. The protein extractabilities of , and were 77.0%, 66.5% and 53.6%, respectively, corresponding to reductions in protein extractability by 10%, 22% and 37% compared to DOWNHT. A similar decrease in protein extractability with increasing oat groat moisture content was observed for , and (Table 1). This consistent trend of decreasing protein extractability with oat groat moisture content was not seen for . For , a significant (P < 0.05) decrease in protein extractability was only observed when the oat groat moisture content was 20.0%, as compared to and . These findings suggest that at 80 and 90 °C, protein extractability losses as function of oat groat moisture content occur only if oat groats are heat treated for at least 30 min. At 100 °C, the DOW protein extractability was significantly (P < 0.05) higher at 13.0% moisture than at 16.5% moisture, irrespective of the heating time, with no further decrease (P > 0.05) observed at 20.0% moisture.
Fig. S2B presents the impact of heating temperature (80, 90 and 100 °C) on the protein extractabilities of DOW obtained from oat groats with a moisture content of 16.5% heat treated for 30 min. As the temperature increased, protein extractability significantly (P < 0.05) decreased. For , a protein extractability of 75.8% was obtained, while showed a much lower extractability of 35.5%. This corresponds to protein extractability reductions of 12% and 59%, respectively, compared to DOWNHT. This trend of decreasing protein extractability with increasing temperature was evident for all combinations of oat groat moisture content and heating time (Table 1).
Fig. S2C shows the impact of varying heating times (15, 30 and 45 min) on the protein extractabilities of DOW obtained from oat groats with a moisture content of 16.5% heat treated at 90 °C. It was observed that increasing the heating time significantly (P < 0.05) reduced protein extractability, with reductions of 17%, 22% and 39% compared to DOWNHT for , and , respectively. A similar decreasing trend in protein extractability with an increase in heating time was noted for , , and (Table 1). The impact of heating time on the protein extractability of and was negligible. Independent of the oat groat moisture content, a significant (P < 0.05) decrease in protein extractability compared to DOWNHT was observed for . Even lower (P < 0.05) protein extractabilities were obtained when the heating time was extended to 30 min. Increasing the heating time from 30 to 45 min did not lead to a further decrease in protein extractability.
Thus, it was observed that both the peroxidase activity (see section 3.1) and the protein extractability (at pH 9.0) decreased with increasing oat groat moisture content, temperature and heating time. Among the different heat treatment conditions, two groups of DOWLHT samples could be distinguished. The first group included DOWLHT samples with higher oat protein extractabilities (52–85%) than DOWIHT (21%) but with residual peroxidase activity (0.7–7.5 U/g dm). This was observed for , regardless of oat groat moisture content and heating time, as well as for samples – with the exception of and . The second group comprised DOWLHT samples with higher oat protein extractabilities than DOWIHT and complete peroxidase inactivation, i.e. , and all samples. These samples had protein extractabilities of 54%, 46% and 31–59%, respectively, compared to DOWIHT (21%). Thus, it is possible to alter the oat groat heat treatment conditions so that peroxidases are fully inactivated, resulting in shelf-stable oat groats, while at the same time achieving protein extractabilities nearly three times higher than observed for DOWIHT.
Based on the obtained results and the available literature, it is highly likely that the industrially produced heat-treated oat groats studied here, were subjected to much more harsh heat treatment conditions than the here applied lab-scale heat treatment conditions. The difference between industrial and here applied lab-scale heat treatments lies in, amongst others, the applied temperature-time combination, mode of the heat treatment (i.e. steaming vs. tempering and heating, respectively) and the scale. Despite these differences, the obtained results for approached those of industrially heat-treated oats, indicating the relevance of our chosen lab-scale heat treatment conditions.
3.3. Impact of a selected set of heat treatment conditions on the lipase activity of defatted oat whole meals
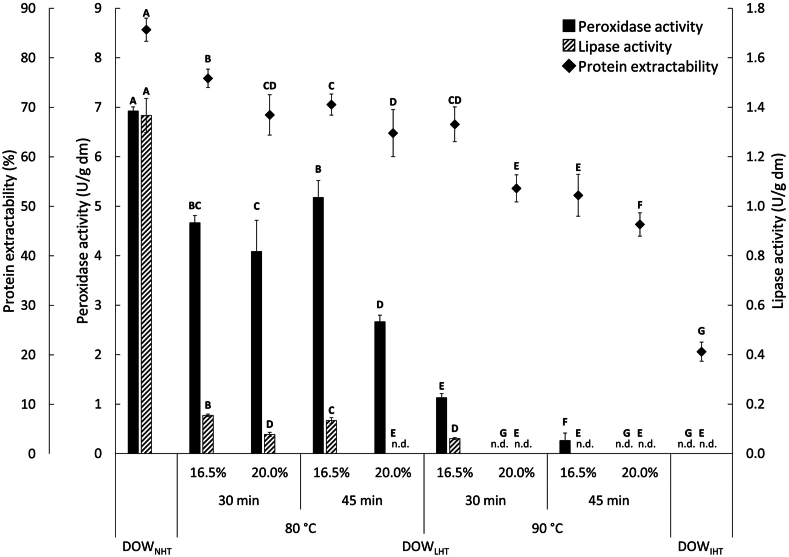
As mentioned previously, peroxidases are the most heat-stable enzymes, and therefore their activity is an often-used indicator for oat groat shelf stability in industry (Ekstrand et al., 1992; Ganẞmann and Vorwerck, 1995). However, it might well be possible that oat lipases can be inactivated under milder heat treatment conditions than required for complete peroxidase inactivation. Applying milder conditions could result in retaining even higher protein extractabilities than concluded in section 3.2. Fig. 2 shows the lipase activities of DOWNHT, DOWIHT and a selected set of DOWLHT samples, compared to their respective peroxidase activities.
Fig. 2.
The protein extractability (diamonds, first left y-axis), peroxidase activity (solid filled bars, second left y-axis) and lipase activity (hatched bars, right y-axis) of defatted oat whole meal (DOW) from non-heat-treated oat groats, industrially heat-treated oat groats and a selected set of lab-scale heat-treated oat groats. For the latter, heat treatment conditions (oat groat moisture content, heating time and heating temperature) are specified. Abbreviations: DOWNHT = DOW from non-heat-treated oat groats, DOWIHT = DOW from industrially heat-treated oat groats, DOWLHT = DOW from lab-scale heat treated oat groats, n.d. = not detected (below detection limit). Diamonds and bars from the same type with a different capital letter are significantly different (α = 0.05). Error bars represent the deviation from the mean of triplicate measurements.
The lipase activity of DOWNHT was 1.40 U/g dm, while for DOWIHT, as for the peroxidase activity, no lipase activity was detected. The lipases of all samples were assumed to be inactivated, which was confirmed by determining the lipase activity of and (data not shown). For and , complete peroxidase and lipase inactivation was observed. From Fig. 2, it is clear that oat lipases are less heat-stable than peroxidases, which was expected and is in line with Ekstrand et al. (1992). For example, for , the peroxidase activity decreased from 6.92 U/g dm (DOWNHT) to 4.66 U/g dm, while the lipase activity decreased from 1.40 U/g dm (DOWNHT) to 0.15 U/g dm. This corresponds to relative reductions in enzyme activities of 33% and 89%, respectively, compared to DOWNHT. Similar trends were observed for and . For and , no lipase activity was detected, while residual peroxidase activities of 2.66 U/g dm and 0.26 U/g dm were observed, respectively. Thus, milder heat treatment conditions can be applied to fully inactivate lipases than those needed for inactivating peroxidases.
Fig. 2 also depicts the protein extractabilities of DOWNHT, DOWIHT and a selected set of DOWLHT samples. The DOWLHT samples for which complete lipase inactivation was observed, i.e. , , , and all samples, had protein extractabilities ranging from 31% to 65%. Within this group, the highest protein extractability of 65% was observed for . In a separate statistical test comparing and , which was identified in section 3.2 as the sample with the highest protein extractability and complete peroxidase inactivation, the former was found to have significantly higher (P < 0.05) protein extractability (65%) than the latter (59%). While this is interesting conceptually, it should be noted that in the cases where lipases are inactivated but residual peroxidase activity remains (i.e. and ), a thorough investigation of the shelf-life of the obtained oat-based products is still deemed necessary.
3.4. The impact of varying heat treatment conditions on the microstructure of oat groats
The residual peroxidase activity and protein extractability of DOW strongly depend on the heat treatment conditions to which oat groats are subjected (Table 1). As these heat treatments are performed on the oat groats prior to milling, it is of interest to investigate the impact of the treatment on the groat microstructure. This has, to the best of our knowledge, not yet been reported on in the available literature. Fig. 3A shows a cross-section of a non-heat-treated oat groat. The outer layers (i.e. pericarp, seed coat and nucellus, O in Fig. 3A) can be observed as compressed layers surrounding the aleurone layer (A in Fig. 3A), which consist of rectangular cells. Below the aleurone layer, towards the interior of the oat groat, the sub-aleurone cells (S in Fig. 3A) and the starchy endosperm (E in Fig. 3A) can be observed. It is clear that proteins – here stained in blue (Fig. 3A) – were distributed throughout the oat groat, with high concentrations in the aleurone cells. Fig. S3A shows that the aleurone cells have cell walls containing low amounts of β-D-glucan – here stained with Calcofluor, to be able to assess the impact of heat treatment on the oat groat cell wall structures – while the sub-aleurone cells have thicker cell walls, containing mostly β-D-glucan. This is in accordance with previous research, in which it was also observed that oat aleurone cell walls predominantly consist of arabinoxylan (Dornez et al., 2011b; Miller and Fulcher, 2011).
Fig. 3.
Light microscopy image of a transverse section of (A) a non-heat-treated oat groat, and (B) an industrially heat-treated oat groat. Proteins (in blue) were stained with Naphthol Blue Black. Abbreviations: O = outer layers, A = aleurone layer, S = sub-aleurone layer, E = starchy endosperm.
Fig. 3B shows the microstructure of an oat groat subjected to industrial heat treatment. As expected, the same cellular tissues (i.e. outer layers, aleurone layer, sub-aleurone layer and starchy endosperm) as in non-heat-treated oat groats could easily be identified (Fig. 3A). The β-D-glucan in the cell walls of these layers was not visibly impacted by the industrial heat treatment (Figure S3B). However, proteins were significantly affected, particularly in the aleurone layer. Indeed, in non-heat-treated oats (Fig. 3A), the proteins present in the aleurone layer are homogeneously distributed within the cells, but in industrially heat-treated oats (Fig. 3B), the intracellular material of the aleurone cells seemed to exhibit clumping. The extensive degree of clumping in the aleurone cells of industrially heat-treated oats, potentially due to protein aggregation, may be associated with the observed protein extractability decrease from 86% for DOWNHT to 21% for DOWIHT. It was argued by Runyon et al. (2015) that the loss in protein extractability was caused by (partial) denaturation and aggregation of proteins upon heat treatment. However, no experimental evidence supporting the proposed hypothesis was presented. The sub-aleurone layer and the starchy endosperm visually seemed unaffected by the industrial heat treatment, where proteins seemed to be evenly distributed within their respective cells (Fig. 3B). However, taking into account that up to 50% of the total protein is present in the bran – containing the aleurone layer – of high-protein oat varieties (Youngs, 1972) and observing a protein extractability loss of 65%, it is highly unlikely that proteins in the sub-aleurone layer and starchy endosperm remain unaffected upon industrially heat treating oats. High-resolution microscopy or other techniques, such as differential scanning calorimetry or secondary structure analysis, might shed even more light on the microstructural organization of (heat-treated) oat groats as well as on the degree of protein denaturation/aggregation therein.
Fig. 4 shows the impact of temperature (80, 90 and 100 °C) on the microstructure of selected lab-scale heat-treated oat groats at fixed moisture content-heating time combinations of 13.0%–15 min and 20.0%-45 min. Proteins present in the aleurone cells of oat groats heat treated at 80 °C (Fig. 4A and 4B) were homogenously distributed, as was the case for non-heat-treated oat groats (Fig. 3A). For and , no or very limited clumping was observed, and the protein extractability only decreased (P < 0.05) by 12% and 24%, respectively, compared to DOWNHT (Table 1). Upon increasing the temperature to 90 or 100 °C, a higher degree of aleurone intracellular material clumping (Fig. 4C-F) and a more pronounced decrease in oat protein extractability (Table 1) was observed. Relative protein extractability reductions of 17%, 46%, 31% and 64% for , , and , respectively, compared to DOWNHT, were observed. It thus seems that clumping of the aleurone intracellular material only occurs at heating temperatures of ≥90 °C, and it appears that this phenomenon is connected with a pronounced reduction in protein extractability. However, as mentioned above, more research is needed to fully grasp the mechanism by which oat heat treatment influences protein extractability. Finally, the lab-scale heat treatments, as was the case for the industrial heat-treatment, did not have a clear visual impact on the β-D-glucan present in the cell walls of the different cellular tissues (Fig. S3).
Fig. 4.
Light microscopy image of a transverse section of an oat groat with (A) 13.0% moisture heat treated at 80 °C for 15 min, (B) 20.0% moisture heat treated at 80 °C for 45 min, (C) 13.0% moisture heat treated at 90 °C for 15 min, (D) 20.0% moisture heat treated at 90 °C for 45 min, (E) 13.0% moisture heat treated at 100 °C for 15 min, (F) 20.0% moisture heat treated at 100 °C for 45 min. Proteins (in blue) were stained with Naphthol Blue Black.
4. Conclusions
Oats are typically heat treated (or ‘kilned’) to inactivate endogenous lipases, thereby preventing lipid hydrolysis and rancidity development. Such heat treatment, however, drastically reduces protein extractability, which limits the nutritional and technological contribution of oat proteins in oat-based dairy alternatives. To the best of our knowledge based on existing literature, this study established for the first time that oat heat treatment conditions can be directed to successfully achieve complete enzyme inactivation while retaining high protein extractability. Several lab-scale heat-treated oats for which complete peroxidase inactivation (a measure for all enzyme inactivation in industry), had significantly higher protein extractabilities (31–59%) than industrially heat-treated oats (21%). When targeting the inactivation of oat lipase (established as less heat-stable enzymes than peroxidases) as an indicator for oat groat shelf stability, milder heat treatment conditions were required, whilst retaining protein extractabilities of 31–65%. In the latter case, further research on the shelf-life stability of oat-based products is still required. Upon heat treating oats (in the industrial process as well as at ≥ 90 °C in the lab-scale process), clumping of the intracellular material of the aleurone cells, potentially due to protein aggregation, was observed, which seemed to be connected to the decrease in protein extractability. More research is needed to fully understand the mechanism by which oat heat treatment influences protein extractability and to investigate to what extent proteins in the aleurone, sub-aleurone and starchy endosperm tissues are affected.
Although further research is needed, our findings have important implications for oat processing companies, which could apply the achieved insights to re-evaluate the industrial heat treatment of oat groats. This could inevitably impact manufacturers of oat-based dairy alternatives, such as drinks and yogurt alternatives, who will be able to produce oat liquid bases with higher protein contents. The potential of oat proteins could hence be better utilized in these types of products, without compromising shelf stability.
CRediT authorship contribution statement
Ines Pynket: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Frederik Janssen: Conceptualization, Methodology, Validation, Supervision, Writing – review & editing, Supervision, Project administration. Jarne Van Gils: Formal analysis, Investigation, Methodology. Christophe M. Courtin: Conceptualization, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition. Arno G.B. Wouters: Conceptualization, Methodology, Validation, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used ChatGPT in order to improve the readability and language of the manuscript. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the published article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Frederik Janssen gratefully acknowledges the Research Foundation – Flanders (FWO Vlaanderen, Brussels, Belgium) for a postdoctoral fellowship (grant 1224123N). FWO Vlaanderen is further acknowledged for providing funding for research project G049824N.
Handling Editor: Professor A.G. Marangoni
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2024.100932.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Replication Data for: Directing oat groat heat treatment conditions towards increased protein extractability https://doi.org/10.48804/UIQQDF.
References
- AACCI . Approved Methods of Analysis. AACC International; 1999. Method 44-19.01. [DOI] [Google Scholar]
- Aiking H., de Boer J. The next protein transition. Trends Food Sci. Technol. 2020;105:515–522. doi: 10.1016/j.tifs.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydar E.F., Tutuncu S., Ozcelik B. Plant-based milk substitutes: bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct.Foods. 2020;70 doi: 10.1016/j.jff.2020.103975. [DOI] [Google Scholar]
- Clark P.L., Plaxco K.W., Sosnick T.R. Water as a good solvent for unfolded proteins: folding and collapse are fundamentally different. J. Mol. Biol. 2020;432(9):2882–2889. doi: 10.1016/j.jmb.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker E.A., Rose D.J., Stewart D.A. Processing of oats and the impact of processing operations on nutrition and health benefits. Br. J. Nutr. 2014;112:S58–S64. doi: 10.1017/S000711451400227X. [DOI] [PubMed] [Google Scholar]
- Dornez E., Cuyvers S., Holopainen U., Nordlund E., Poutanen K., Delcour J.A., Courtin C.M. Inactive fluorescently labeled xylanase as a novel probe for microscopic analysis of arabinoxylan containing cereal cell walls. J. Agric. Food Chem. 2011;59(12):6369–6375. doi: 10.1021/jf200746g. [DOI] [PubMed] [Google Scholar]
- Dornez E., Holopainen U., Cuyvers S., Poutanen K., Delcour J.A., Courtin C.M., Nordlund E. Study of grain cell wall structures by microscopic analysis with four different staining techniques. J. Cereal. Sci. 2011;54(3):363–373. doi: 10.1016/j.jcs.2011.07.003. [DOI] [Google Scholar]
- Ekstrand B., Gangby I., Akesson G. Lipase activity in oats: distribution, pH dependence and heat inactivation. Cereal Chem. 1992;69(4):379–381. [Google Scholar]
- Ganẞmann W., Vorwerck K. In: The Oat Crop: Production and Utilization. Welch R.W., editor. Springer; Dordrecht: 1995. Oat milling, processing and storage; pp. 369–408. [DOI] [Google Scholar]
- Gates F.K., Sontag-Strohm T., Stoddard F.L., Dobraszczyk B.J., Salovaara H. Interaction of heat-moisture conditions and physical properties in oat processing: II. Flake quality. J. Cereal. Sci. 2008;48(2):288–293. doi: 10.1016/j.jcs.2007.09.009. [DOI] [Google Scholar]
- Girardet N., Webster F.H. In: Oats: Chemistry and Technology. second ed. Webster F.H., Wood P.J., editors. AACC International, Inc; 2011. Oat milling: specifications, storage, and processing; pp. 301–319. [DOI] [Google Scholar]
- Hermans W., Mutlu S., Michalski A., Langenaeken N.A., Courtin C.M. The contribution of sub-aleurone cells to wheat endosperm protein content and gradient is dependent on cultivar and N-fertilization level. J. Agric. Food Chem. 2021;69(23):6444–6454. doi: 10.1021/acs.jafc.1c01279. [DOI] [PubMed] [Google Scholar]
- Hutchinson J.B., Martin H.F., Moran T. Location and destruction of lipase in oats. Nature. 1951;167:758–759. doi: 10.1038/167758a0. [DOI] [PubMed] [Google Scholar]
- Janssen F., Lambrechts E., Pynket I., Wouters A.G.B. Ball milling alters the extractability and colloidal state of oat proteins. J. Cereal. Sci. 2023;112 doi: 10.1016/j.jcs.2023.103725. [DOI] [Google Scholar]
- Klose C., Arendt E.K. Proteins in oats; their synthesis and changes during germination: a review. Crit. Rev. Food Sci. Nutr. 2012;52(7):629–639. doi: 10.1080/10408398.2010.504902. [DOI] [PubMed] [Google Scholar]
- Lásztity R. Oat grain - a wonderful reservoir of natural nutrients and biologically active substances. Food Rev. Int. 1998;14(1):99–119. doi: 10.1080/87559129809541150. [DOI] [Google Scholar]
- Lehtinen P., Kiiliäinen K., Lehtomäki I., Laakso S. Effect of heat treatment on lipid stability in processed oats. J. Cereal. Sci. 2003;37(2):215–221. doi: 10.1006/jcrs.2002.0496. [DOI] [Google Scholar]
- Lehtinen P., Laakso S. Role of lipid reactions in quality of oat products. Agric. Food Sci. 2004;13(1–2):88–99. doi: 10.2137/1239099041838085. [DOI] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. doi: 10.1016/s0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Ma C.Y., Harwalkar V.R. Thermal coagulation of oat globulin. Cereal Chem. 1987;64(4):212–218. [Google Scholar]
- Mäkinen O.E., Sozer N., Ercili-Cura D., Poutanen K. In: Sustainable Protein Sources. Nadathur S.R., Wanasundara J.P.D., Scanlin L., editors. Academic Press; 2017. Protein from oat: structure, processes, functionality, and nutrition; pp. 105–119. [DOI] [Google Scholar]
- Mariotti F., Tomé D., Mirand P.P. Converting nitrogen into protein - beyond 6.25 and Jones' factors. Crit. Rev. Food Sci. Nutr. 2008;48(2):177–184. doi: 10.1080/10408390701279749. [DOI] [PubMed] [Google Scholar]
- McClements D.J., Grossmann L. A brief review of the science behind the design of healthy and sustainable plant-based foods. Sci. Food. 2021;5(17) doi: 10.1038/s41538-021-00099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements D.J., Newman E., McClements I.F. Plant-based milks: a review of the science underpinning their design, fabrication, and performance. Compr. Rev. Food Sci. Food Saf. 2019;18(6):2047–2067. doi: 10.1111/1541-4337.12505. [DOI] [PubMed] [Google Scholar]
- Miller S.S., Fulcher R.G. In: Oats: Chemistry and Technology. second ed. Webster F.H., Wood P.J., editors. AACC International, Inc; 2011. Microstructure and chemistry of the oat kernel; pp. 77–94. [DOI] [Google Scholar]
- Montemurro M., Pontonio E., Coda R., Rizzello C.G. Plant-based alternatives to yogurt: state-of-the-art and perspectives of new biotechnological challenges. Foods. 2021;10(2):1–21. doi: 10.3390/foods10020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pålsson D., Penttinen H., Malmberg C., Adlercreutz P., Tullberg C. Rancidity development in oat during industrial processing. LWT. 2024;204 doi: 10.1016/j.lwt.2024.116448. [DOI] [Google Scholar]
- Pütter J. In: Methods of Enzymatic Analysis. 2. Bergmeyer H.U., editor. Academic Press; 1974. Peroxidases; pp. 685–690. vol. 2. [DOI] [Google Scholar]
- Runyon J.R., Sunilkumar B.A., Nilsson L., Rascon A., Bergenståhl B. The effect of heat treatment on the soluble protein content of oats. J. Cereal. Sci. 2015;65:119–124. doi: 10.1016/j.jcs.2015.06.008. [DOI] [Google Scholar]
- Sim S.Y.J., Srv A., Chiang J.H., Henry C.J. Plant proteins for future foods: a roadmap. Foods. 2021;10(8):1–31. doi: 10.3390/foods10081967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaen J., Silva J.V.C. Oat proteins: review of extraction methods and techno-functionality for liquid and semi-solid applications. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2021;147 doi: 10.1016/j.lwt.2021.111478. [DOI] [Google Scholar]
- Sunilkumar B.A., Leonova S., Öste R., Olsson O. Identification and characterization of high protein oat lines from a mutagenized oat population. J. Cereal. Sci. 2017;75:100–107. doi: 10.1016/j.jcs.2017.03.003. [DOI] [Google Scholar]
- Wei C.Y., Hund A., Zhu D., Nyström L. Exploring genetic dependence of lipase activity to improve the quality of whole-grain wheat. J. Sci. Food Agric. 2020;100(7):3120–3125. doi: 10.1002/jsfa.10346. [DOI] [PubMed] [Google Scholar]
- Welch R.W. In: Oats: Chemistry and Technology. second ed. Webster F.H., Wood P.J., editors. AACC International, Inc; 2011. Nutrient composition and nutritional quality of oats and comparisons with other cereals; pp. 95–107. [DOI] [Google Scholar]
- Yang Z., Piironen V., Lampi A.-M. Lipid-modifying enzymes in oat and faba bean. Food Res. Int. 2017;100:335–343. doi: 10.1016/j.foodres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Youngs V.L. Protein distribution in the oat kernel. Cereal Chem. 1972;49:407–411. [Google Scholar]
- Zang J., Yan B., Hu H., Liu Z., Tang D., Liu Y., Chen J., Tu Y., Yin Z. The current advances, challenges, and future trends of plant-based yogurt. Trends Food Sci. Technol. 2024;149 doi: 10.1016/j.tifs.2024.104531. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Replication Data for: Directing oat groat heat treatment conditions towards increased protein extractability https://doi.org/10.48804/UIQQDF.