Abstract
Background: Congenital hypothyroidism (CH) is a common metabolic disorder in children that can impact growth and neurodevelopment, particularly during infancy and early childhood. DUOXA2, a DUOX maturation factor, plays a crucial role in the maturation and activation of dual oxidase DUOX2 (a member of the NADPH oxidase family). DUOX2 can correctly migrate to the plasma membrane from the endoplasmic reticulum (ER) with the help of DUOXA2, and the two proteins together form a stable complex that promotes hydrogen peroxide (H2O2) generation in the synthesis of thyroid hormones. Genetic alterations in DUOXA2 lead to defects function of DUOX2 protein causing inherited CH.
Objectives: This review discusses the relationship between DUOXA2 and CH, including the pathogenic mechanisms of CH in children caused by DUOXA2 mutations and the possibility or promise of DUOXA2 gene screening as a diagnostic marker for CH in the clinic.
Methods: The review synthesizes current research on the biological role of DUOXA2 and DUOX2 in thyroid hormone synthesis, the molecular impact of DUOXA2 mutations, and the clinical implications of genetic screening for CH.
Results: Mutations in DUOXA2 disrupt this process of H2O2 generation in the synthesis of thyroid hormones , leading to inherited CH. Early identification through DUOXA2 gene screening could improve diagnostic accuracy, which facilitates early intervention and personalized treatment.
Conclusions: DUOXA2 gene screening holds promise for enhancing diagnostic accuracy in CH. However, it cannot be used as a sole diagnostic indicator, and to optimize diagnostic sensitivity, it should be combined with the screening of other relevant genetic mutations and diagnostic tools. Further research is needed to refine screening protocols and explore therapeutic options.
Keywords: Congenital hypothyroidism, NADPH oxidase, H2O2, DUOX2, DUOXA2
1. Introduction
CH is one of the most prevalent hereditary endocrine illnesses, characterized by high thyrotropin hormone (TSH) and decreased blood thyroxine (T4) levels, with 1 in 3,000 babies suffering from congenital hypothyroidism [1]. Facial mucous oedema, macroglossia, umbilical hernia, hypotonia, expanded fontanelle, enlarged belly, reduced activity and increased drowsiness, constipation, feeding problems, and persistent jaundice are some of the more common signs [2]. Clinically, primary CH is caused by thyroid dysplasia or thyroid dyshormonogenesis, whereas secondary or central CH is caused by TSH shortage [3]. According to recent research, the percentage of CH brought on by thyroid dyshormonogenesis is rising [4]. Thyroid hormones are required for appropriate growth, development, and metabolism, and CH can cause growth retardation and neurodevelopmental issues if left untreated [5,6]. The causes of thyroid hormone synthesis disorders typically include non-genetic factors, such as inadequate iodine intake, as well as genetic factors, such as mutations or deletions in genes encoding key proteins involved in the synthesis process [7].
Gene alterations that encode key enzymes or transport proteins involved in thyroid hormone synthesis, such as iodine organication disorders (TPO, DUOX2, DUOXA2, SLC26A4), [8] defective thyroglobulin (TG) [9] synthesis or transport, iodine transport defects (SLC5A5), and iodotyrosine deiodinase (DEHAL1), are typically causes of thyroid hormone synthesis disorders [10,11]. The majority of gene mutations causing CH have long been identified and discussed. However, mutations in DUOXA2, which were first associated with CH in 2008, are relatively rare, and there is less discussion on this topic. In 2008, Zamproni et al. [12] discovered that mutations in the dual oxidase maturation factor 2 (DUOXA2) gene can disrupt thyroid hormone production, which is important in the development of CH. The link between DUOXA2 and CH has also drawn a lot of interest.
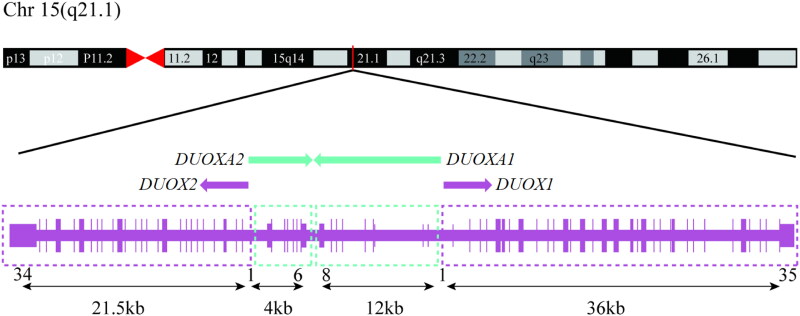
The dual oxidases DUOX1 and DUOX2 are nicotinamide adenine dinucleotide phosphate (NADPH) oxidases involved in the synthesis of H2O2 [13]. With a length of 1,551 and 1,548 amino acids, respectively, the DUOX1 and DUOX2 proteins have 83% sequence identity [14]. DUOX1 and DUOX2 can form heterodimers with the particular maturation factors DUOXA1 and DUOXA2, respectively, and these heterodimers are necessary for the DUOXs enzyme complex’s maturity and functionality [15]. While the DUOXA2 gene has six exons and encodes 320 amino acids with five transmembrane segments, the DUOXA1 open reading frame spans 11 exons and generates a 483 amino acid protein [16]. The DUOX1 and DUOX2 genes are situated on chromosome 15 (15q21.1) and are transcribed in opposite orientations with the DUOXA1 and DUOXA2 genes positioned head-to-head in the spacer area between the two DUOX genes (Figure 1) [17]. The co-expression of the DUOX/DUOXA complex is caused by the bidirectional core promoter region shared by each DUOXA gene and its corresponding chaperone DUOX genes. DUOX2 is thought to be the dominant isoenzyme in the thyroid, as evidenced by its higher thyroidal expression levels and mutations in the human DUOX2 and DUOXA2 genes have been related to temporary or persistent CH, according to some clinical researches [18–20]. The DUOX2 enzyme generates H2O2, a crucial electron acceptor for the thyroid peroxidase–catalysed iodination and coupling reactions mediating thyroid hormone biosynthesis [21]. Genetic defects in DUOXA2 may lead to defective DUOX2 protein function, which results in compromised H2O2 production. This ultimately impacts the iodination of thyroglobulin, which is mediated by thyroid peroxidase (TPO), resulting in inadequate thyroid hormone synthesis [22]. In addition to regulating thyroid hormone synthesis, DUOXA2 regulates oxidative stress by modulating H2O2 production which in turn affects cell proliferation and apoptosis [23]. For instance, overexpression of DUOXA2 can lead to excessive H2O2 production, which may result in DNA damage [24]. Furthermore, DUOXA2’s regulation of H2O2 extends beyond cellular survival, playing a crucial role in immune modulation. Elevated H2O2 levels serve as key signalling molecules that regulate the activation, differentiation, and proliferation of immune cells [25]. This modulation shapes the inflammatory response and enhances the organism’s capacity to combat infections [26]. Since DUOX1 is also involved in the thyroid gland’s production of H2O2, there has been speculation that DUOX1 may experience fluctuating upregulation in order to supplement the deficiencies of DUOX2 [24]. Nevertheless, mutations in the DUOX1/DUOXA1 complex have not yet been linked to any of the documented cases of CH [27].
Figure 1.
Schematic presentation of the DUOX1/2 and DUOXA1/2 genes locus showing the orientation of the genes on the long arm of chromosome 15. Created with Adobe Illustrator.
2. DUOXA2 mutations in congenital hypothyroidism
2.1. DUOXA2 is involved in thyroid hormone biosynthesis
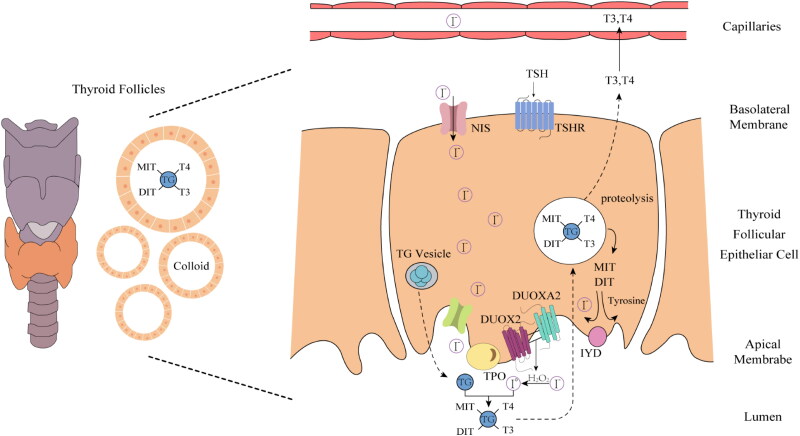
The intricate biochemical process of thyroid hormone synthesis involves iodide uptake, iodination, iodinated tyrosine coupling, and finally, the synthesis of thyroid hormones and their release into the bloodstream. This process takes place in the intersection of the cytoplasm and apical membrane of thyroid follicular cells (Figure 2).
Figure 2.
Schematic Illustrating the critical molecules involved in thyroid hormone biosynthesis. Thyroid hormone biosynthesis requires a complex pathway of enzymes and transporter molecules permitting uptake, concentration, and organification of circulating iodide, as well as TG substrate for iodination. Pathogenic variants in genes encoding these components (TSHR, NIS, TG, TPO, DUOX2, DUOXA2, IYD) may result in CH, and mutations in the NADPH-oxidase DUOX2 and its accessory protein DUOXA2 are particularly implicated in CH. Created with Adobe Illustrator.
1. Iodide Uptake: The synthesis of thyroid hormones relies on iodide uptake. Thyrocytes absorb iodide from the blood via iodide transporters. This process, known as iodide ‘capture,’ is a critical step in the synthesis of thyroid hormones. 2. Iodination Process: A significant amount of circulating iodinated substrate and thyroglobulin (TG) are required for iodination, which is TPO-catalysed except for the step of iodine polymerization. The process is mediated by the sodium-iodide symporter (NIS) molecule and enzymes expressed by thyroid follicular cells. When the TPO catalysed process reaches the final electron acceptor stage, H2O2 serves as an oxidizing agent [28]. 3. Coupling Reaction: In the presence of H2O2, iodide undergoes oxidation and attaches itself to tyrosine residues on the TG surface to generate monoiodotyrosine (MIT) and diiodotyrosine (DIT). These two compounds are then dual-coupled to produce thyroid hormones (T3 and T4) [29]. 4. Release of Thyroid Hormones: Once synthesis is complete, T3 and T4 remain bound within the thyroglobulin. Through endocytosis, thyrocytes reabsorb the thyroglobulin-containing granules, which are then degraded in lysosomes, releasing free T3 and T4. 5. Entry into the Bloodstream: The free T3 and T4 are released into the bloodstream. In the blood, these hormones bind to thyroid hormone-binding proteins, such as thyroxine-binding globulin, forming stable complexes that facilitate their effective transport to various tissues throughout the body [30].
The involvement of H2O2 in the aforementioned thyroid hormone synthesis is critical because it stimulates thyroid hormone synthesis by pushing TPO to catalyse the reaction of iodinated tyrosine [31]. Any factors that affect the production and utilization of H2O2 may affect the normal synthesis of thyroid hormones, and thus have an important impact on the normal function of the body, leading to the occurrence of CH [15]. Studies have shown that the primary enzyme that supplies H2O2 to TPO is a heterodimer composed of DUOX2 and DUOXA2, located on the top membrane of the thyroid follicular cells, which belongs to the NADPH oxidase compound [32]. In addition to the catalytic core, which includes NADPH- and FAD-binding sites and a haeme arrangement for electron transfer from NADPH to O2, the DUOX2 protein features a N-terminal extracellular peroxidase homologous domain [33]. This domain serves as the binding site for TPO and maturation factors, which are essential for H2O2 production and substrate engagement [34]. This is followed by a membrane-spanning region containing six transmembrane segments that facilitate anchoring in the plasma membrane, ensuring structural stability and efficient electron transfer [35]. Together, these structural domains enable DUOX2 to effectively participate in redox signalling and immune responses. DUOX2 is crucial for the rate-limiting stage of thyroid hormone synthesis, known as iodination process, which is catalysed by TPO [36]. DUOX2 can create H2O2 directly or by using the creation of O2- as a precursor. Two methods are available for moving iodide from the extracellular space to the follicular lumen: passive transport via the parietal membrane and active transport via the basolateral plasma membrane. Iodide is translocated into the follicular lumen along with TG once it enters the cell, where it is oxidized by DUOX2-TPO at the apical membrane. Immunoprecipitation investigations have revealed that DUOX and TPO are strongly coupled to the cell membrane and that their binding can be up-regulated by the Gq-phospholipase C-Ca2+-protein kinase C (Gq-PLC) pathway and down-regulated by the Gs-cAMP-protein kinase A (Gs-PKA) pathway [37]. The activity of DUOX2 is also influenced by the local level of H2O2, which is stimulated at low concentrations and inhibited at high concentrations [38]. As a DUOX2 maturation factor, DUOXA2 plays an important role in the maturation and activation of DUOX2 and is involved in the transport of DUOX2 from the endoplasmic reticulum (ER) to the plasma membrane. The N-terminal domain of DUOXA2 facilitates DUOX2’s membrane localization and is critical for proper functioning [39]. An extracellular loop between the second and third transmembrane regions contains three conserved N-glycosylation sites vital for protein maturation [40]. Furthermore, the extracellular loop between the first and second transmembrane segments is crucial for stabilizing the complex with DUOX2. However, the functions of the C-terminal cytoplasmic region of DUOXA2 remain largely uncharacterized, necessitating further research to explore its regulatory potential. DUOXA2 not only allows for the rapid exit of correctly folded DUOX2 proteins from the ER and their translocation to the plasma membrane of the cell, and it also contributes to the degradation of incorrectly folded DUOX2 proteins [41]. These two proteins are expressed in the parietal membrane near the TPO and interact with each other. Genetic defects in DUOXA2 may lead to secondary defects in DUOX2 activity, resulting in retention of the oxidase in the endoplasmic reticulum and reduced release of H2O2, thereby affecting thyroid hormone synthesis [42].
2.2. DUOXA2 gene related Congenital hypothyroidism
Research has indicated that the DUOXA2 gene may cause CH through either a single allele mutation or a double allele variation [43]. There are many phenotypes associated with CH, ranging from moderate subclinical with high TSH levels and normal thyroid hormone levels to dominant CH with impaired thyroid hormone production [44].
Initially, Zamproni et al. [12] 2008 identified a nonsense mutation c.738C > G (p.Y246X) in the DUOXA2 gene in a patient with mild permanent CH and hormone synthesis-impaired Goiter (Table 1). In the study, one heterozygous carrier of Y246X was first identified among 92 Chinese individuals. Additionally, further genetic testing of clinical cases indicated that this type of homozygous mutation is the most common DUOXA2 mutation in the Chinese population. This mutation led to the deletion of DUOXA2’s transmembrane helix 5 and its C-terminal cytoplasmic structural domain, which manifests the mutant protein subject to rapid degradation after synthesis, resulting in lower steady-state expression. This ultimately resulted in the complete loss of the patient’s functional DUOXA2 protein and DUOX2 activity. These findings provide in vivo evidence of the crucial role DUOXA2 plays in thyroid hormone synthesis. In 2011, Hulur et al. [45] reported a novel DUOXA2 missense mutation (p.C189R), associated with a complete impairment of H2O2 generation, was identified in compound heterozygosis with a large deletion encompassing DUOX2 and DUOXA2 in a patient with transient CH. This mutation introduces a charged residue into the third transmembrane helix of the DUOXA2 protein, resulting in an unstable protein that is rapidly degraded and does not allow the reconstitution of detectable NADPH oxidase activity. Subsequently, in 2013, Yi et al [46] reported for the first time the insertion of the code-shift mutation c.413-414insA (p.Y138X). Among the 47 patients in this study, the researchers found a code-shift mutation in only one patient. This mutation resulted in an early termination of protein translation due to a premature TAA stop codon, which shortened the DUOXA2 protein by 183 amino acids. In 2015, Liu et al. [43] identified a novel heterozygous missense mutation, c.C78G (p.I26M), and a homozygous nonsense mutation, c.C738G (p.Y246X) of DUOXA2 in 75 CH patients with mild transient and mild permanent Goiter, respectively. This novel heterozygous missense mutation caused an alteration in the signal peptide region of the DUOXA2 gene, which may affect the correct folding and transport of the DUOXA2 protein, thereby impacting its function. Functional experiments showed that it led to aberrant H2O2 synthesis and thus CH. Park et al. [47] conducted genetic screening in 58 outpatients with confirmed primary CH. They found two cases of compound heterozygous mutations carrying both c.413-414insA (p.Y138X) mutation and other allelic mutations of DUOXA2 manifesting as transient CH in 2016. Tan et al. [48] detected six cases of DUOXA2 mutation in 20 children with CH, none of them had a family history of thyroid-related diseases. Among the 20 patients, two had p.Y246X/p.Y246X homozygous mutation; four had monoallelic heterozygous mutation, among whom two carried the known pathogenic mutation p.Y138X, one carried p.Y246X, and one carried a novel mutation, p.G79R. The novel mutation c.235G > C (p.G79R) altered the extracellular domain of the DUOXA2, leading to impaired function of the DUOXA2 protein. This amino acid substitution could interfere with the interaction between DUOXA2 and DUOX2, thereby impacting the synthesis and secretion of thyroid hormones. 2019 Peter et al. [49] identified four cases of DUOXA2 single allele mutations (p.E128 *, p.V78M, N121 _ E122delinsK) and two cases of compound heterozygous mutations in performing early screening for newborns with CH.
Table 1.
DUOXA2 Mutation in CH.
| Nucleotide position | Amino acid position | Mutation type | References |
|---|---|---|---|
| c.738C > G | p.Y246X | nonsense mutation | Zamproni et al. (2008) [12] |
| c.565T > C | p.C189R | missense mutation | Hulur et al. (2011) [45] |
| c.413-414insA | p.Y138X | code-shift mutation | Yi et al. (2013) [46] |
| c.78C > G | p.126M | missense mutation | Liu et al. (2013) [50] |
| c.413-414insA | p.Y138X | code-shift mutation | Park et al. (2016) [47] |
| c.235G > C | p.G79R | missense mutation | Tan et al. (2017) [48] |
| c.382G > T | p.E128* | nonsense mutation | Peter et al. (2019) [49] |
| c.363_365delCGA | p.N121_E122delinsK | insertion/deletion mutation | Peter et al. (2019) [49] |
| c.790G > C | p.G264R | missense mutation | Peter et al. (2019) [49] |
| c.893_894delTT | p.L298Hfs*21 | frameshift mutation | Peter et al. (2019) [49] |
| c.232G > A | p.V78M | missense mutation | Peter et al. (2019) [49] |
| c.228G > T | p.W76C | missense mutation | Peter et al. (2019) [49] |
| c.611T > C | p.L204P | missense mutation | Peter et al. (2019) [49] |
In conclusion, DUOXA2 gene mutations can lead to structural changes that may disrupt the interaction with DUOX2, or result in functional inactivation. This impairment can prevent DUOX2 from being transported to the plasma membrane and synthesizing H2O2, which is crucial for the production of thyroid hormones.
3. Early clinical screening of CH
To guarantee that all newborns receive medication in a timely manner, congenital hypothyroidism screening is mandated by legislation and regulations in many nations and regions [50]. Thyroid hormone levels, or TSH, are typically measured in the blood of infants as part of early screening [51]. This is a rather easy, affordable, and practical screening procedure that may be carried out very soon after birth. Intravenous thyroid function tests are used to confirm the diagnosis of CH when increased TSH or abnormal thyroxine (T4) levels are detected (this depends on the area of the newborn screening program) [52]. The European Society of Pediatric Endocrinology (ESPE) suggests rescreening all preterm children in addition to heel blood screening in order to identify the specific form of CH defined by delayed TSH elevation of the serum [53]. Moreover, problems in the synthesis of thyroid hormone and thyroid hypoplasia can be detected using thyroid imaging [54]. However, in certain cases, maternal thyroid hormones may be transferred to the foetus via the placenta, temporarily masking the symptoms of neonatal hypothyroidism [55]. This implies that although the levels of thyroid hormones in the neonate may be low, there may be an absence of overt clinical manifestations immediately following birth. CH resulting from DUOX2 and DUOXA2 mutations typically manifests as reduced thyroid hormone levels during the neonatal period.55 These patients may exhibit normal clinical presentation at birth, as their symptoms might not be detected promptly during newborn screening [56]. Research indicates that routine newborn screening (NBS) results in certain instances may yield negative findings despite mutations in DUOX2/DUOXA2 [57]. The presence of mutations in the DUOXA2 gene, which may serve as the reference for diagnosis and enable the early diagnosis or pre-symptomatic diagnosis of CH. [58] Genetic testing can identify mutations in DUOXA2, thereby facilitating the diagnosis of CH [30]. Furthermore, genetic testing of family members with a DUOXA2 mutation can inform family planning and genetic counselling by assessing their risk of developing the condition [59]. This testing can be integrated with routine assessments of thyroid hormone levels in clinical practice to improve diagnostic accuracy [60]. Based on current clinical data, the probability of CH due to DUOXA2 mutations is lower compared to DUOX2 mutations [29]. Consequently, screening for a single mutation is insufficient for diagnosing CH, necessitating a combined multigene analysis. Considering the results of the latest studies, genetic testing and further analysis can be used not only to reveal the aetiology of CH but also to determine the subsequent treatment course and predict prognosis [61]. Genetic testing and traditional early screening methods have their own advantages and disadvantages, and the combination of the two may produce the effect of one plus one more than two.
4. Treatment and intervention
In healthy newborns, assessment of thyroid function, including TSH and free T4 (FT4) measurements, is usually recommended every 1 to 2 weeks. Primary CH is diagnosed when TSH secretion exceeds or FT4 synthesis falls below the age-specific reference interval, and pharmacologic therapy is recommended [62]. Thyroid hormone replacement therapy is the major treatment for CH caused by mutations in the DUOXA2 gene, which mostly manifests as reduced thyroid hormone synthesis. This helps to support normal growth and development and helps correct metabolism. Early detection and prompt treatment of CH are essential to optimizing neurocognitive outcomes, linear growth, pubertal growth and progression, and eventual height [63]. Early intervention is therefore essential in CH caused by DUOXA2 mutations. The treatment of choice after diagnosis of CH is levothyroxine (L-T4), with a starting dose of 10 to 15 μg/kg per day administered once daily. If enteral administration is not possible, L-T4 can be given intravenously at 75% of the enteral dose [64]. In addition to conventional hormone replacement therapy, which remains the cornerstone of treatment for paediatric CH, the emergence of gene therapy presents a possible new therapy, particularly for patients with mutations in genes related to thyroid hormone synthesis, like DUOXA2. This therapeutic strategy targets the genetic defect at its source, potentially enabling the restoration of normal thyroid function through the introduction of functional genes or the correction of faulty ones [65]. Recent advancements in molecular genetics have facilitated the development of personalized treatment regimens, meticulously tailored to individual mutation profiles [66]. This precision medicine approach allows clinicians to fine-tune hormone replacement dosages based on specific genetic alterations, thereby optimizing serum thyroid hormone levels and ensuring adequate metabolic support [67]. For example, patients with varying degrees of DUOXA2 mutations may require distinct dosing strategies that account for their unique physiological responses. Moreover, ongoing monitoring of therapeutic efficacy is crucial. Regular assessments of thyroid function tests can guide healthcare providers in adjusting both the dosage and duration of hormone therapy, catering to the dynamic needs of each patient. Such individualized management not only enhances treatment effectiveness but also minimizes potential side effects, which can arise from suboptimal dosing [68].
In conclusion, the success of treatment depends on timely diagnosis, individualization of the treatment plan, and patient cooperation.
5. Conclusions and future perspectives
DUOXA2 is involved in the transport of DUOX2 from the ER to the plasma membrane, and the two protein together form a stable complex here to promote H2O2 synthesis, crucial for thyroid hormone regulation. The DUOX2/DUOXA2 heterodimer exhibits abnormal functionality due to mutations in the DUOXA2 gene, leading to reduced catalytic activity of TPO and impaired H2O2 synthesis, ultimately affecting thyroid hormone production and causing CH. Thus, genetic testing for these mutations is crucial for precise diagnosis. Early screening of DUOXA2 mutations can significantly improve patient outcomes through timely L-T4 therapy, supporting normal growth and neurocognitive development. Future research should focus on innovative therapeutic approaches and further understanding the molecular mechanisms of DUOXA2-related CH, aiming to develop tailored interventions and improve clinical management for affected patients.
The combination of traditional thyroid function tests with genetic diagnostics offers a more comprehensive approach to the clinical evaluation of paediatric CH [69]. Conventional screening methods, such as measuring serum thyroid hormone levels and utilizing imaging techniques, remain fundamental for early detection. However, given the genetic heterogeneity of CH, traditional diagnostics alone often fall short in detecting the underlying molecular causes. By integrating genetic testing for DUOXA2 mutations, clinicians can enhance diagnostic accuracy and enable more targeted management strategies. This dual approach not only aids in confirming the diagnosis but also provides valuable information for family planning and genetic counselling. The implementation of multigenic screening for CH presents significant opportunities and challenges [70]. A more extensive sample size is necessary to establish robust associations between specific mutations and the prevalence of the condition. Ideally, researchers should develop a predictive model that correlates identified mutations with disease incidence, which would facilitate earlier diagnosis and intervention. This model would help quantify the contribution of various mutations to the overall pathogenesis, thereby offering clinicians a more accurate means of disease prediction and aiding in the differentiation of pathogenic variants from benign ones.
While the potential for gene therapy targeting DUOXA2 or other thyroid-related genes is substantial, numerous challenges persist. First and foremost, the safety and efficacy of gene therapies must be rigorously validated through clinical trials. Moreover, effective and precise delivery of therapeutic genes to the target cells remains a significant hurdle. Researchers are tasked with developing reliable vectors that can achieve efficient gene transfer while minimizing off-target effects. Additionally, controlling the expression levels of the introduced genes is crucial to avoid unintended consequences. Addressing these challenges is imperative for translating the promise of gene therapy into viable treatment options for patients with congenital hypothyroidism linked to DUOXA2 mutations.
Acknowledgements
Not applicable.
Funding Statement
This study was supported by National Natural Science Foundation of China (82360223) and Research Project of Yan’an University (205040404, D2022144).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
J.D. and Y.Y. conceived the idea of deliberation and were responsible for drafting this manuscript, and X.Z. made an important contribution to revising the ideological content of this manuscript. D.W., J.W., C.T. Q.H., H.B. and C.C. participated in overall supervision. X.Z. reviewed and edited. All authors give final approval of the version to be published and give an agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data availability is not applicable to this article as no new data were created or analyzed in this review.
References
- 1.Kwak MJ. Clinical genetics of defects in thyroid hormone synthesis. Ann Pediatr Endocrinol Metab. 2018;23(4):169–175. doi: 10.6065/apem.2018.23.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kostopoulou E, Miliordos K, Spiliotis B, et al. Genetics of primary congenital hypothyroidism-a review. Hormones (Athens). 2021;20(2):225–236. doi: 10.1007/s42000-020-00267-x. [DOI] [PubMed] [Google Scholar]
- 3.Léger J, Olivieri A, Donaldson M, et al. European society for paediatric endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99(2):363–384. doi: 10.1210/jc.2013-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemipour M, Ghasemi M, Hovsepian S, et al. Etiology of congenital hypothyroidism in isfahan: does it different? Adv Biomed Res. 2014;3:21. doi: 10.4103/2277-9175.124658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitropoulos A, Molinari L, Etter K, et al. Children with congenital hypothyroidism: long-term intellectual outcome after early high-dose treatment. Pediatr Res. 2009;65(2):242–248. doi: 10.1203/PDR.0b013e31818d2030. [DOI] [PubMed] [Google Scholar]
- 6.Grosse SD, Van Vliet G.. Prevention of intellectual disability through screening for congenital hypothyroidism: how much and at what level? Arch Dis Child. 2011;96(4):374–379. doi: 10.1136/adc.2010.190280. [DOI] [PubMed] [Google Scholar]
- 7.Moran C, Schoenmakers N, Visser WE, et al. Genetic disorders of thyroid development, hormone biosynthesis and signalling. Clin Endocrinol (Oxf). 2022;97(4):502–514. doi: 10.1111/cen.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Zang Y, Li M, et al. Duox2 and duoxa2 variants confer susceptibility to thyroid dysgenesis and gland-in-situ with congenital hypothyroidism. Front Endocrinol (Lausanne). 2020;11:237. doi: 10.3389/fendo.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targovnik HM, Citterio CE, Rivolta CM, et al. Iodide handling disorders (nis, tpo, tg, iyd). Best Pract Res Clin Endocrinol Metab. 2017;31(2):195–212. doi: 10.1016/j.beem.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Leung AKC, Leung AAC.. Evaluation and management of the child with hypothyroidism. World J Pediatr. 2019;15(2):124–134. doi: 10.1007/s12519-019-00230-w. [DOI] [PubMed] [Google Scholar]
- 11.Zhou D, Yang R, Huang X, et al. Results of neonatal screening for congenital hypothyroidism and hyperphenylalaninemia in zhejiang province from 1999 to 2022. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023;52(6):683–692. doi: 10.3724/zdxbyxb-2023-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamproni I, Grasberger H, Cortinovis F, et al. Biallelic inactivation of the dual oxidase maturation factor 2 (duoxa2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008;93(2):605–610. doi: 10.1210/jc.2007-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donkó Á, Morand S, Korzeniowska A, et al. Hypothyroidism-associated missense mutation impairs nadph oxidase activity and intracellular trafficking of duox2. Free Radic Biol Med. 2014;73:190–200. doi: 10.1016/j.freeradbiomed.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neill S, Brault J, Stasia M-J, et al. Genetic disorders coupled to ros deficiency. Redox Biol. 2015;6:135–156. doi: 10.1016/j.redox.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasberger H. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol Cell Endocrinol. 2010;322(1-2):99–106. doi: 10.1016/j.mce.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Grasberger H, Refetoff S.. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006;281(27):18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 17.Christophe-Hobertus C, Christophe D.. Delimitation and functional characterization of the bidirectional thox-duoxa promoter regions in thyrocytes. Mol Cell Endocrinol. 2010;317(1-2):161–167. doi: 10.1016/j.mce.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Baz-Redón N, Antolín M, Clemente M, et al. Patients with thyroid dyshormonogenesis and duox2 variants: molecular and clinical description and genotype-phenotype correlation. Int J Mol Sci. 2024;25(15):8473. doi: 10.3390/ijms25158473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uehara E, Hori N, Tanase-Nakao K, et al. Congenital hypothyroidism with thyroid in situ: a case report with nkx2-1 and duox2 hypomorphic variants. Horm Res Paediatr. 2024:1–7. (2024). doi: 10.1159/000538895. [DOI] [PubMed] [Google Scholar]
- 20.Aycan Z, Cangul H, Muzza M, et al. Digenic duox1 and duox2 mutations in cases with congenital hypothyroidism. J Clin Endocrinol Metab. 2017;102(9):3085–3090. doi: 10.1210/jc.2017-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivolta CM, Louis-Tisserand M, Varela V, et al. Two compound heterozygous mutations (c.215dela/c.2422t–>c and c.387delc/c.1159g–>a) in the thyroid peroxidase gene responsible for congenital goitre and iodide organification defect. Clin Endocrinol (Oxf). 2007;67(2):238–246. doi: 10.1111/j.1365-2265.2007.02869.x. [DOI] [PubMed] [Google Scholar]
- 22.De Deken X, Wang D, Many MC, et al. Cloning of two human thyroid cdnas encoding new members of the nadph oxidase family. J Biol Chem. 2000;275(30):23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 23.Szanto I, Pusztaszeri M, Mavromati M, et al. H(2)o(2) metabolism in normal thyroid cells and in thyroid tumorigenesis: focus on nadph oxidases. Antioxidants (Basel). 2019;8(5):126. doi: 10.3390/antiox8050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poncelet L, Dumont J-E, Miot F, et al. The dual oxidase duox2 stabilized with duoxa2 in an enzymatic complex at the surface of the cell produces extracellular h(2)o(2) able to induce DNA damage in an inducible cellular model. Exp Cell Res. 2019;384(1):111620. doi: 10.1016/j.yexcr.2019.111620. [DOI] [PubMed] [Google Scholar]
- 25.De Deken X, Corvilain B, Dumont JE, et al. Roles of duox-mediated hydrogen peroxide in metabolism, host defense, and signaling. Antioxid Redox Signal. 2014;20(17):2776–2793. doi: 10.1089/ars.2013.5602. [DOI] [PubMed] [Google Scholar]
- 26.Taylor JP, Tse HM.. The role of nadph oxidases in infectious and inflammatory diseases. Redox Biol. 2021;48:102159. doi: 10.1016/j.redox.2021.102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashtiwi NM, Sarr D, Rada B, et al. Duox1 in mammalian disease pathophysiology. J Mol Med (Berl). 2021;99(6):743–754. doi: 10.1007/s00109-021-02058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshihara A, Hara T, Kawashima A, et al. Regulation of dual oxidase expression and h2o2 production by thyroglobulin. Thyroid. 2012;22(10):1054–1062. doi: 10.1089/thy.2012.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters C, Schoenmakers N.. Mechanisms in endocrinology: the pathophysiology of transient congenital hypothyroidism. Eur J Endocrinol. 2022;187(2):R1–r16. doi: 10.1530/eje-21-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters C, van Trotsenburg ASP, Schoenmakers N, et al. Diagnosis of endocrine disease: congenital hypothyroidism: update and perspectives. Eur J Endocrinol. 2018;179(6):R297–r317. doi: 10.1530/eje-18-0383. [DOI] [PubMed] [Google Scholar]
- 31.Weber G, Rabbiosi S, Zamproni I, et al. Genetic defects of hydrogen peroxide generation in the thyroid gland. J Endocrinol Invest. 2013;36(4):261–266. doi: 10.3275/8847. [DOI] [PubMed] [Google Scholar]
- 32.De Deken X, Miot F.. Duox defects and their roles in congenital hypothyroidism. Methods Mol Biol. 2019;1982:667–693. doi: 10.1007/978-1-4939-9424-3_37. [DOI] [PubMed] [Google Scholar]
- 33.Louzada RA, Corre R, Ameziane-El-Hassani R, et al. Conformation of the n-terminal ectodomain elicits different effects on duox function: a potential impact on congenital hypothyroidism caused by a h(2)o(2) production defect. Thyroid. 2018;28(8):1052–1062. doi: 10.1089/thy.2017.0596. [DOI] [PubMed] [Google Scholar]
- 34.Miot F, et al. In: Pick Edgar (editor). Nadph oxidases revisited: from function to structure. Switzerland: Springer International Publishing; 2023.229–245. [Google Scholar]
- 35.Ameziane-El-Hassani R, Schlumberger M, Dupuy C, et al. Nadph oxidases: new actors in thyroid cancer? Nat Rev Endocrinol. 2016;12(8):485–494. doi: 10.1038/nrendo.2016.64. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Liu W, Zhang L, et al. Identification and analyzes of duox2 mutations in two familial congenital hypothyroidism cases. Endocrine. 2021;72(1):147–156. doi: 10.1007/s12020-020-02437-8. [DOI] [PubMed] [Google Scholar]
- 37.Song Y, Ruf J, Lothaire P, et al. Association of duoxes with thyroid peroxidase and its regulation in thyrocytes. J Clin Endocrinol Metab. 2010;95(1):375–382. doi: 10.1210/jc.2009-1727. [DOI] [PubMed] [Google Scholar]
- 38.Morand S, Ueyama T, Tsujibe S, et al. Duox maturation factors form cell surface complexes with duox affecting the specificity of reactive oxygen species generation. Faseb J. 2009;23(4):1205–1218. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leto TL, Morand S, Hurt D, et al. Targeting and regulation of reactive oxygen species generation by nox family nadph oxidases. Antioxid Redox Signal. 2009;11(10):2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohda A, Kamakura S, Hayase J, et al. The nadph oxidases duox1 and duox2 are sorted to the apical plasma membrane in epithelial cells via their respective maturation factors duoxa1 and duoxa2. Genes Cells. 2024;29(10):921–930. doi: 10.1111/gtc.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Targovnik HM, Scheps KG, Rivolta CM, et al. Defects in protein folding in congenital hypothyroidism. Mol Cell Endocrinol. 2020;501:110638. doi: 10.1016/j.mce.2019.110638. [DOI] [PubMed] [Google Scholar]
- 42.Muzza M, Fugazzola L.. Disorders of. Best Pract Res Clin Endocrinol Metab. 2017;31(2):225–240. doi: 10.1016/j.beem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Liu L, Niu X, et al. A novel missense mutation (i26m) in duoxa2 causing congenital goiter hypothyroidism impairs nadph oxidase activity but not protein expression. J Clin Endocrinol Metab. 2015;100(4):1225–1229. doi: 10.1210/jc.2014-3964. [DOI] [PubMed] [Google Scholar]
- 44.Long W, Guo F, Yao R, et al. Genetic and phenotypic characteristics of congenital hypothyroidism in a chinese cohort. Front Endocrinol (Lausanne). 2021;12:705773. doi: 10.3389/fendo.2021.705773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulur I, Hermanns P, Nestoris C, et al. A single copy of the recently identified dual oxidase maturation factor (duoxa) 1 gene produces only mild transient hypothyroidism in a patient with a novel biallelic duoxa2 mutation and monoallelic duoxa1 deletion. J Clin Endocrinol Metab. 2011;96(5):E841–845. doi: 10.1210/jc.2010-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi R-H, Zhu W-B, Yang L-Y, et al. A novel dual oxidase maturation factor 2 gene mutation for congenital hypothyroidism. Int J Mol Med. 2013;31(2):467–470. doi: 10.3892/ijmm.2012.1223. [DOI] [PubMed] [Google Scholar]
- 47.Park K-J, Park H-K, Kim Y-J, et al. Duox2 mutations are frequently associated with congenital hypothyroidism in the korean population. Ann Lab Med. 2016;36(2):145–153. doi: 10.3343/alm.2016.36.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan MY, et al. characteristics of duoxa2 gene mutation in children with congenital hypothyroidism. ]. Zhongguo Dang Dai er ke za Zhi = Chinese Journal of Contemporary Pediatrics. 2017;19:59–63. [. doi: 10.7499/j.issn.1008-8830.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters C, Nicholas AK, Schoenmakers E, et al. Duox2/duoxa2 mutations frequently cause congenital hypothyroidism that evades detection on newborn screening in the united kingdom. Thyroid. 2019;29(6):790–801. doi: 10.1089/thy.2018.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu L, He W, Zhu J, et al. Global prevalence of congenital hypothyroidism among neonates from 1969 to 2020: a systematic review and meta-analysis. Eur J Pediatr. 2023;182(7):2957–2965. doi: 10.1007/s00431-023-04932-2. [DOI] [PubMed] [Google Scholar]
- 51.Boelen A, Zwaveling-Soonawala N, Heijboer AC, et al. Neonatal screening for primary and central congenital hypothyroidism: is it time to go Dutch? Eur Thyroid J. 2023;12(4):e230041. doi: 10.1530/etj-23-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Trotsenburg P, Stoupa A, Léger J, et al. Congenital hypothyroidism: a 2020-2021 consensus guidelines update-an endo-european reference network initiative endorsed by the european society for pediatric endocrinology and the european society for endocrinology. Thyroid. 2021;31(3):387–419. doi: 10.1089/thy.2020.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung HR. Screening and management of thyroid dysfunction in preterm infants. Ann Pediatr Endocrinol Metab. 2019;24(1):15–21. doi: 10.6065/apem.2019.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oron T, Lazar L, Ben-Yishai S, et al. Permanent vs transient congenital hypothyroidism: assessment of predictive variables. J Clin Endocrinol Metab. 2018;103(12):4428–4436. doi: 10.1210/jc.2018-00362. [DOI] [PubMed] [Google Scholar]
- 55.Zdraveska N, Kocova M.. Thyroid function and dysfunction in preterm infants-challenges in evaluation, diagnosis and therapy. Clin Endocrinol (Oxf). 2021;95(4):556–570. doi: 10.1111/cen.14481. [DOI] [PubMed] [Google Scholar]
- 56.Wang F, Xiaole L, Ma R, et al. Dual oxidase system genes defects in children with congenital hypothyroidism. Endocrinology. 2021;162(8):bqab043. doi: 10.1210/endocr/bqab043. [DOI] [PubMed] [Google Scholar]
- 57.Sorapipatcharoen K, Tim-Aroon T, Mahachoklertwattana P, et al. Duox2 variants are a frequent cause of congenital primary hypothyroidism in Thai patients. Endocr Connect. 2020;9(11):1121–1134. doi: 10.1530/ec-20-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun F, Zhang J-X, Yang C-Y, et al. The genetic characteristics of congenital hypothyroidism in china by comprehensive screening of 21 candidate genes. Eur J Endocrinol. 2018;178(6):623–633. doi: 10.1530/eje-17-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugisawa C, Higuchi S, Takagi M, et al. Homozygous duoxa2 mutation (p.Tyr138(*)) in a girl with congenital hypothyroidism and her apparently unaffected brother: case report and review of the literature. Endocr J. 2017;64(8):807–812. doi: 10.1507/endocrj.EJ16-0564. [DOI] [PubMed] [Google Scholar]
- 60.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the american association of clinical endocrinologists and the american thyroid association. Endocr Pract. 2012;18(6):988–1028. doi: 10.4158/ep12280.Gl. [DOI] [PubMed] [Google Scholar]
- 61.Park J, Joo EY, Yoo MJ, et al. Clinical efficacy of multigene panels in the management of congenital hypothyroidism with gland in situ. Medicine (Baltimore). 2024;103(29):e38976. doi: 10.1097/md.0000000000038976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wassner AJ. Congenital hypothyroidism. Clin Perinatol. 2018;45(1):1–18. doi: 10.1016/j.clp.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Delvecchio M, Vigone MC, Wasniewska M, et al. Final height in italian patients with congenital hypothyroidism detected by neonatal screening: a 20-year observational study. Ital J Pediatr. 2015;41(1):82. doi: 10.1186/s13052-015-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El-Kenawy A, Benarba B, Neves AF, et al. Gene surgery: potential applications for human diseases. Excli J. 2019;18:908–930. doi: 10.17179/excli2019-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Persani L, Rurale G, de Filippis T, et al. Genetics and management of congenital hypothyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32(4):387–396. doi: 10.1016/j.beem.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 67.El-Hassani FZ, Fatih F, Joudar N-E, et al. Deep multilayer neural network with weights optimization-based genetic algorithm for predicting hypothyroid disease. Arab J Sci Eng. 2024;49(9):11967–11990. doi: 10.1007/s13369-023-08511-3. [DOI] [Google Scholar]
- 68.Rose SR, Wassner AJ, Wintergerst KA, et al. Congenital hypothyroidism: screening and management. Pediatrics. 2023;151(1):e2022060420. doi: 10.1542/peds.2022-060420. [DOI] [PubMed] [Google Scholar]
- 69.Hanley P, Lord K, Bauer AJ, et al. Thyroid disorders in children and adolescents: a review. JAMA Pediatr. 2016;170(10):1008–1019. doi: 10.1001/jamapediatrics.2016.0486. [DOI] [PubMed] [Google Scholar]
- 70.Nicholas AK, Serra EG, Cangul H, et al. Comprehensive screening of eight known causative genes in congenital hypothyroidism with gland-in-situ. J Clin Endocrinol Metab. 2016;101(12):4521–4531. doi: 10.1210/jc.2016-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this review.