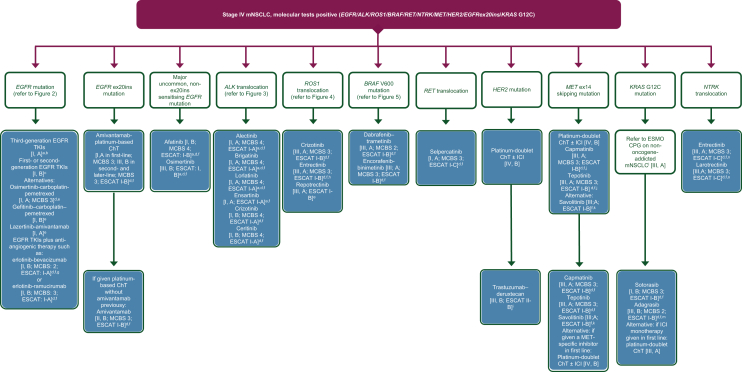
Figure 1.
Treatment algorithm for stage IV mNSCLC after positive findings on molecular tests. Purple: general categories or stratification; blue: systemic anticancer therapy. ChT, chemotherapy; CPG, Clinical Practice Guideline; EGFR, epidermal growth factor receptor; EMA, European Medicines Agency; ESCAT, ESMO Scale for Clinical Actionability of molecular Targets; FDA, Food and Drug Administration; ICI, immune checkpoint inhibitor; MCBS, ESMO-Magnitude of Clinical Benefit Scale; mNSCLC, metastatic non-small-cell lung cancer; TKI, tyrosine kinase inhibitor. aPreferred option(s). bAn example of an approved third-generation TKI is osimertinib [I, A; ESMO-Magnitude of Clinical Benefit (ESMO-MCBS) v1.1 score: 4; ESCAT: I-A]. Lazertinib is another third-generation EGFR TKI that has been approved in Korea for the first-line treatment of patients with EGFR mutations. cExamples of approved first- and second-generation TKIs include erlotinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], gefitinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], afatinib [I, B; ESMO-MCBS v1.1 score: 5; ESCAT: I-A] and dacomitinib [I, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-A]. dESMO-MCBS v1.1 was used to calculate scores for new therapies/indications approved by the EMA or FDA. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).35eNot EMA approved. fESCAT scores apply to alterations from genomic-driven analyses only.36 These scores were defined by the ESMO CPG guideline authors and validated by the ESMO Translational Research and Precision Medicine Working Group.34gEMA approved, not FDA approved. hPreferred over crizotinib in patients with brain metastases. iFDA approved; application for EMA approval withdrawn by the manufacturer. jFDA approved; not EMA approved in first line. kNot EMA or FDA approved. lA parallel ESMO CPG on non-oncogene-addicted mNSCLC is available at: https://www.esmo.org/guidelines/guidelines-by-topic/lung-and-chest-tumours.99mFDA approved; not EMA approved. nIf the patient has not been treated previously with a medicine that works in the same way as entrectinib. oFor patients who have no satisfactory alternative treatments.