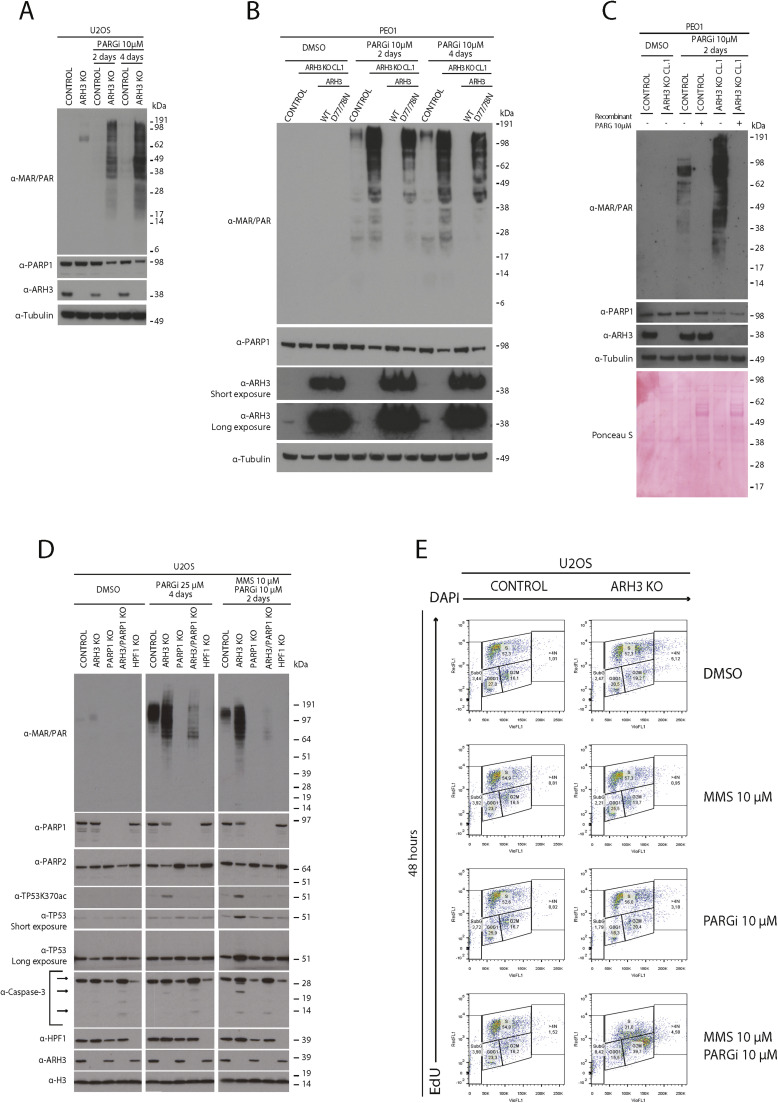
Fig. 5.
Dual ARH3 and PARG enzymatic activity loss correlates with decreased PARP1/2 protein levels. (A) Representative western blot of total cell lysates extracted from parental and ARH3 KO U2OS cells treated with DMSO or 10 µM PARGi for two or four days. α-tubulin served as loading controls. (B) Representative western blot of total cell lysates extracted from parental PEO1, ARH3 KO PEO1, and ARH3 KO PEO1 complemented with wild-type ARH3 (ARH3 WT) or ARH3 D77/78N double mutant (D77/78N) treated with DMSO or 10 µM PARGi for two or four days. α-tubulin served as loading controls. (C) Representative western blotting analysis of total cell lysates extracted from control and ARH3 KO PEO1. The cells were treated with DMSO or 10 μM PARGi for two days. The cells were lysed, and total cell extracts were incubated with or without 10 μM recombinant PARG enzyme for 30 minutes at 30°C. The cell lysates were then analyzed by western blotting using the indicated antibodies. α-tubulin and Ponceau S served as loading controls. (D) Representative western blot of total cell lysates extracted from parental and ARH3 KO, PARP1 KO, ARH3/PARP1 double KO, and HPF1 KO U2OS cells treated with DMSO or 25 µM PARGi for four days or with the combination of MMS and 10 µM PARGi for two days. α-H3 served as loading controls. (E) Representative scatterplots of flow cytometry analysis for cells were treated with DMSO, MMS, PARGi or a combination of MMS and PARGi for 48 hours and stained with EdU-DAPI. Experiments in this figure were performed in biological triplicates.