Abstract
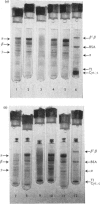
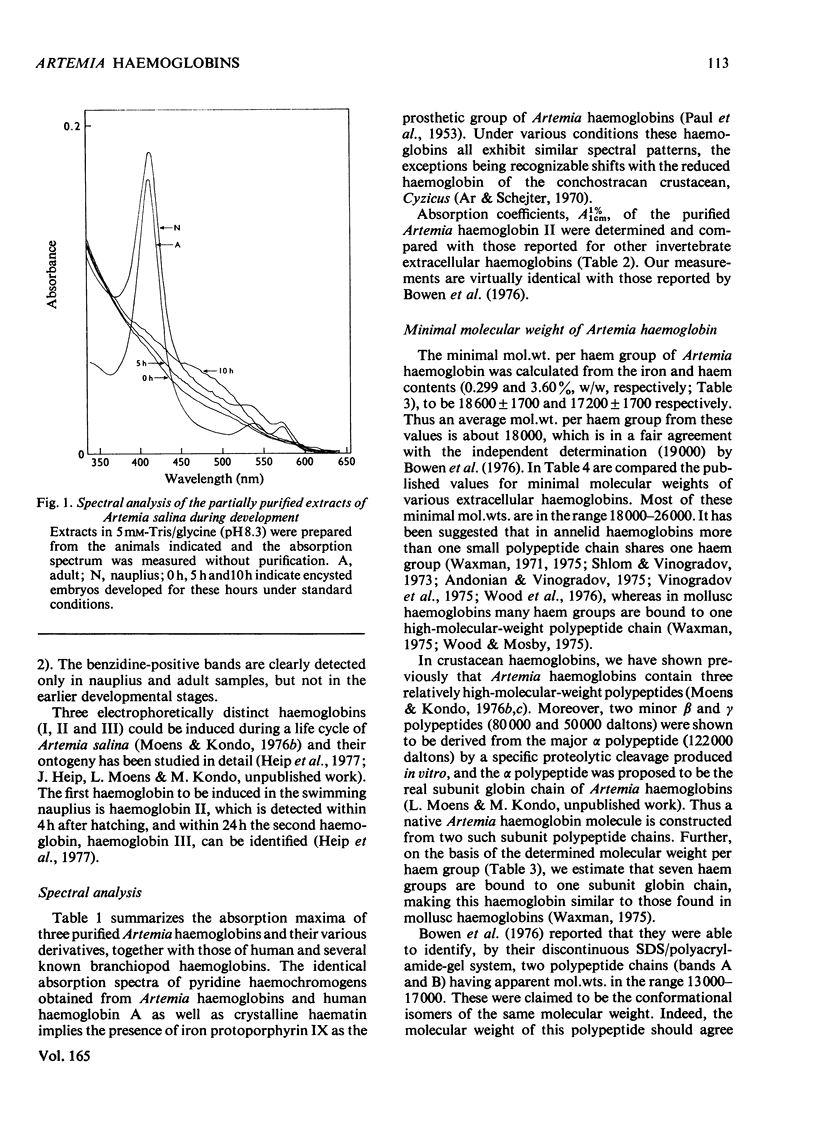
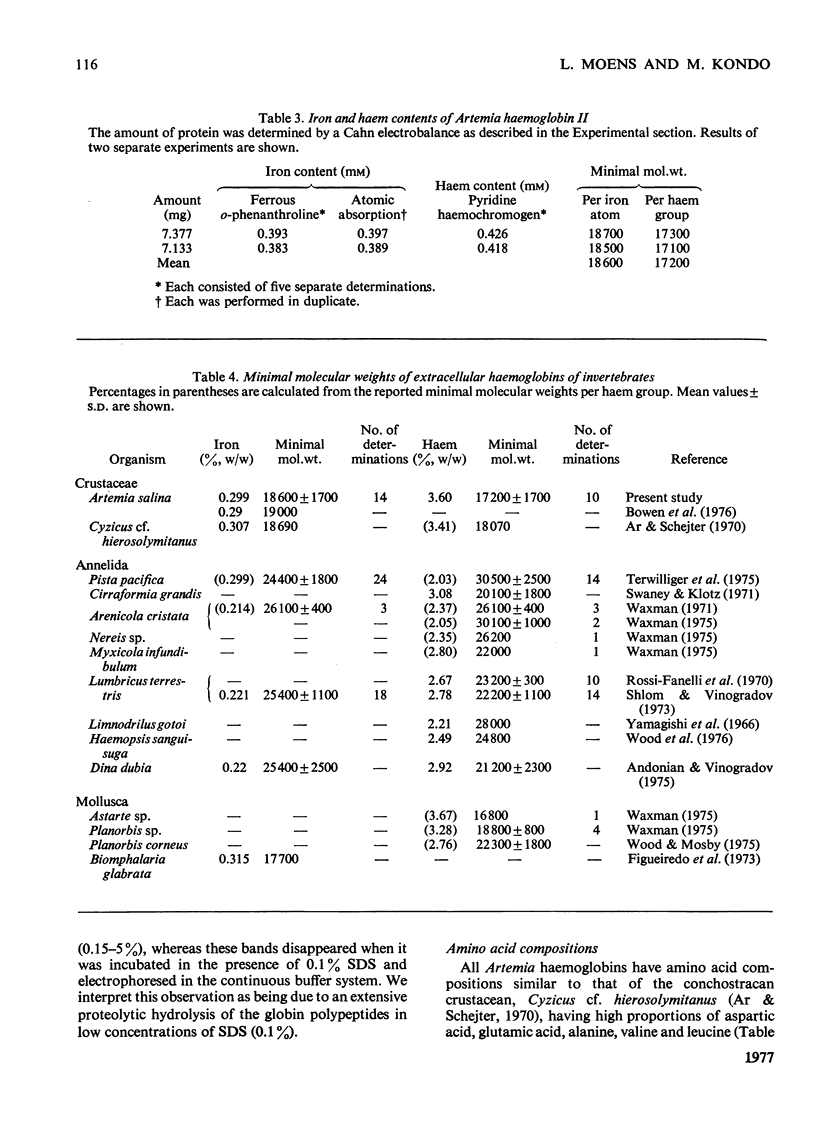
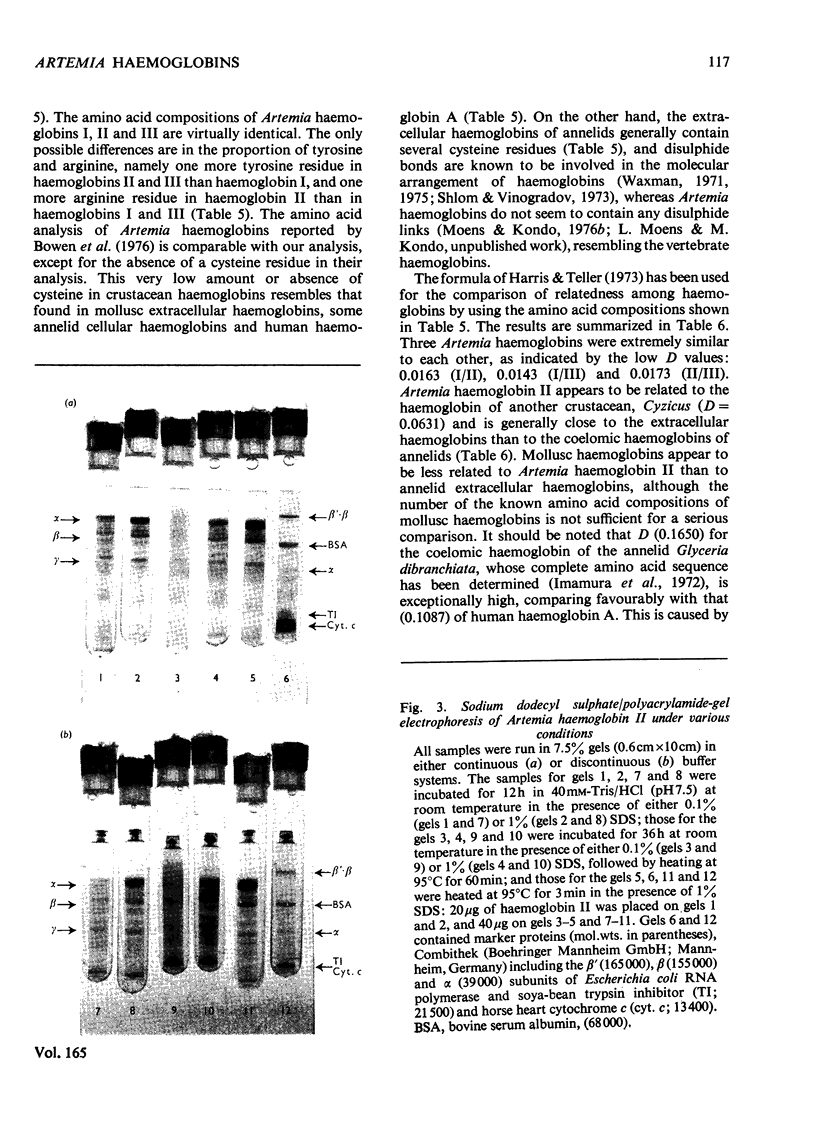
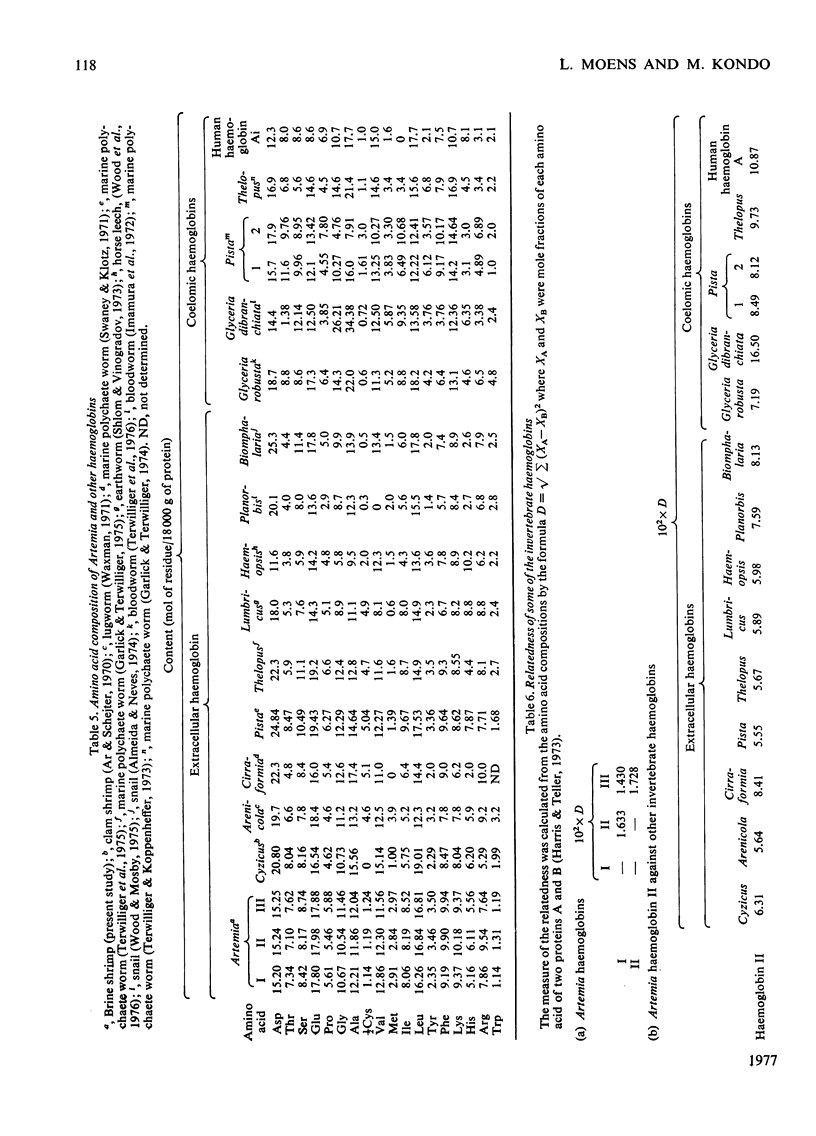
The following factors were measured for extracellular haemoglobins of Artemia salina: a minimal molecular weight of globin chain per haem group (based on the iron and haem contents), the absorption coefficients, the absorption spectra of various derivatives and the amino acid compositions. These were compared with those of the haemoglobins of other invertebrates. Three Artemia haemoglobins (I, II and III) had similar molecular structures, constructed from two-globin subunits of 122000-130000mol.wt. Since the minimal mol.wt. was determined to be 18000, this suggests that one globin subunit was bound by seven haem groups, and hence one haemoglobin molecule (240000-260000mol.wt.) should contain 14 haem groups. A successful identification of this high-molecular-weight subunit required first the denaturation of haemoglobin in 1% sodium dodecyl sulphate before sodium dodecyl sulphate gel electrophoresis. Denaturation by prolonged incubation (12-36 h) at room temperature in the presence of 0.1% sodium dodecyl sulphate [Bowen, Moise, Waring & Poon (1976) Comp. Biochem. Physiol. B55, 99-103] was accompanied by extensive proteolysis, resulting in low recovery of the stainable protein and heterogeneous gel patterns. Regardless of which electrophoretic system was used, the high-molecular-weight subunit was always present provided that 1% sodium dodecyl sulphate was present during denaturation. These results contrast with those obtained by Bowen et al. (1976). However, preferential cleavage of the globin subunit (alpha) seemed to occur in vitro when standard conditions were used, producing two specific fragments having mol.wts. of 80000 (beta) and 50000 (gamma).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida A. P., Neves A. G. The hemoglobin of Biomphalaria glabrata: chemical composition and some physicochemical properties. Biochim Biophys Acta. 1974 Nov 5;371(1):140–146. doi: 10.1016/0005-2795(74)90162-7. [DOI] [PubMed] [Google Scholar]
- Andonian M. R., Vinogradov S. N. Physical properties and subunits of DNA dubia erythrocruorin. Biochim Biophys Acta. 1975 Aug 19;400(2):244–254. doi: 10.1016/0005-2795(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Ar A., Schejter A. Isolation and properties of the hemoglobin of the clam shrimp Cyzicus cf. hierosolymitanus (S. Fischer). Comp Biochem Physiol. 1970 Apr 1;33(3):481–490. doi: 10.1016/0010-406x(70)90365-8. [DOI] [PubMed] [Google Scholar]
- Bowen S. T., Moise H. W., Waring G., Poon M. C. The hemoglobins of Artemia salina--III. Characterization. Comp Biochem Physiol B. 1976;55(1):99–103. doi: 10.1016/0305-0491(76)90180-2. [DOI] [PubMed] [Google Scholar]
- Braun V., Crichton R. R., Barunitzer G. Hämoglobine. XV. Uber monomere und dimere Insektenhämoglobine (Chironomus thummi) Hoppe Seylers Z Physiol Chem. 1968 Feb;349(2):197–210. [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- Cameron B. F. Determination of iron in heme compounds. II. Hemoglobin and myoglobin. Anal Biochem. 1965 May;11(2):164–169. doi: 10.1016/0003-2697(65)90002-3. [DOI] [PubMed] [Google Scholar]
- David M. M., Daniel E. Subunit structure of earthworm erythrocruorin. J Mol Biol. 1974 Jul 25;87(1):89–101. doi: 10.1016/0022-2836(74)90561-0. [DOI] [PubMed] [Google Scholar]
- Garlick R. L., Terwilliger R. C. Coelomic cell hemoglobin of the terebellid polychaete, Thelepus crispus johnson. Structure and oxygen equilibrium. Comp Biochem Physiol B. 1974 Mar 15;47(3):543–553. doi: 10.1016/0305-0491(74)90003-0. [DOI] [PubMed] [Google Scholar]
- Garlick R. L., Terwilliger R. C. The quaternary structura and oxygen equilibrium properties of the vascular hemoglobin of the terebellid polychaete, Thelepus crispus Johnson. Comp Biochem Physiol A Comp Physiol. 1975 Aug 1;51(4):849–857. doi: 10.1016/0300-9629(75)90065-1. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Harris C. E., Teller D. C. Estimation of primary sequence homology from amino acid composition of evolutionary related proteins. J Theor Biol. 1973 Feb;38(2):347–362. doi: 10.1016/0022-5193(73)90179-3. [DOI] [PubMed] [Google Scholar]
- Imamura T., Baldwin T. O., Riggs A. The amino acid sequence of the monomeric hemoglobin component from the bloodworm, Glyat liver. J Biol Chem. 1972 May 10;247(9):2785–2797. [PubMed] [Google Scholar]
- Kamen R., Kondo M., Römer W., Weissmann C. Reconstitution of Q replicase lacking subunit with protein-synthesis-interference factor i. Eur J Biochem. 1972 Nov 21;31(1):44–51. doi: 10.1111/j.1432-1033.1972.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W. Protein polypeptide chain molecular weights by gel chromatography in guanidinium chloride. Methods Enzymol. 1972;26:28–42. doi: 10.1016/s0076-6879(72)26004-9. [DOI] [PubMed] [Google Scholar]
- Marrink J., Gruber M. Molecular weight determination by chromatography on sepharose 4B. FEBS Lett. 1969 Feb;2(4):242–244. doi: 10.1016/0014-5793(69)80031-1. [DOI] [PubMed] [Google Scholar]
- Moens L., Kondo M. Large subunit contained in the extracellular haemoglobins of Artemia salina [proceedings]. Arch Int Physiol Biochim. 1976 Dec;84(5):1096–1098. [PubMed] [Google Scholar]
- Moens L., Kondo M. Polysome-dependent synthesis of embryonic proteins of Artemia salina during cell differentiation and analysis of heme-containing protein. Dev Biol. 1976 Apr;49(2):457–469. doi: 10.1016/0012-1606(76)90187-1. [DOI] [PubMed] [Google Scholar]
- Moens L., Kondo M. The structure of Artemia salina haemoglobins. A comparative characterisation of four naupliar and adult heamoglobins. Eur J Biochem. 1976 Aug 16;67(2):397–402. doi: 10.1111/j.1432-1033.1976.tb10704.x. [DOI] [PubMed] [Google Scholar]
- Rossi Fanelli M. R., Chiancone E., Vecchini P., Antonini E. Studies on erythrocruorin. I. Physicochemical properties of earthworm erythrocruorin. Arch Biochem Biophys. 1970 Nov;141(1):278–283. doi: 10.1016/0003-9861(70)90133-5. [DOI] [PubMed] [Google Scholar]
- Shlom J. M., Vinogradov S. N. A study of the subunit structure of the extracellular hemoglobin of Lumbricus terrestris. J Biol Chem. 1973 Nov 25;248(22):7904–7912. [PubMed] [Google Scholar]
- Swaney J. B., Klotz I. M. Properties of erythrocruorin from Cirraformia grandis. Arch Biochem Biophys. 1971 Dec;147(2):475–486. doi: 10.1016/0003-9861(71)90404-8. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Terwilliger R. C., Garlick R. L., Terwilliger N. B. Hemoglobins of Glycera robusta: structures of coelomic cell hemoglobin and body wall myoglobin. Comp Biochem Physiol B. 1976;54(1):149–153. doi: 10.1016/0305-0491(76)90073-0. [DOI] [PubMed] [Google Scholar]
- Terwilliger R. C., Terwilliger N. B., Roxby R. Quaternary structure of Pista pacifica vascular hemoglobin. Comp Biochem Physiol B. 1975 Feb 15;50(2B):225–232. doi: 10.1016/0305-0491(75)90267-9. [DOI] [PubMed] [Google Scholar]
- Vinogradov S. N., Hersey S. L., Shukuya R. Subunits of Limnodrilus erythrocruorin. Biochem Biophys Res Commun. 1975 Sep 16;66(2):505–513. doi: 10.1016/0006-291x(75)90539-2. [DOI] [PubMed] [Google Scholar]
- Waxman L. The hemoglobin of Arenicola cristata. J Biol Chem. 1971 Dec 10;246(23):7318–7327. [PubMed] [Google Scholar]
- Waxman L. The structure of annelid and mollusc hemoglobins. J Biol Chem. 1975 May 25;250(10):3790–3795. [PubMed] [Google Scholar]
- Wood E. J., Mosby L. J. Physicochemical properties of Planorbis corneus erythrocruorin. Biochem J. 1975 Aug;149(2):437–445. doi: 10.1042/bj1490437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. J., Mosby L. J., Robinson M. S. Characterization of the extracellular haemoglobin of Haemopsis sanguisuga (L.). Biochem J. 1976 Mar 1;153(3):589–596. doi: 10.1042/bj1530589. [DOI] [PMC free article] [PubMed] [Google Scholar]