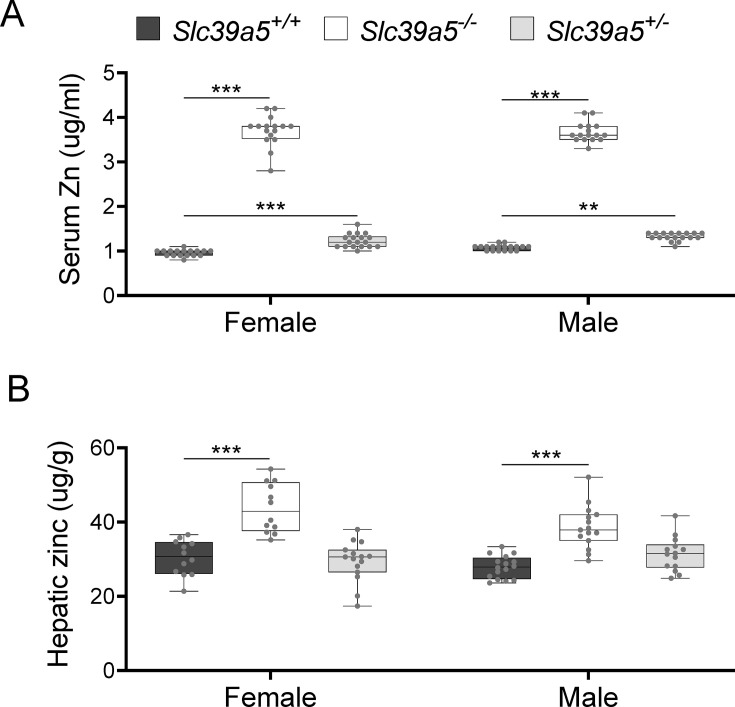
Figure 2. Loss of Slc39a5 results in elevated circulating and hepatic zinc levels in mice.
Serum zinc (A) and hepatic zinc (B) in Slc39a5+/+, Slc39a5-/-, and Slc39a5+/-mice at 40 wk of age, n=16–18. **p<0.01, ***p<0.001, two-way ANOVA with post hoc Tukey’s test.