Fig. 1. |. Overview of the concept and protocol of termini restraining.
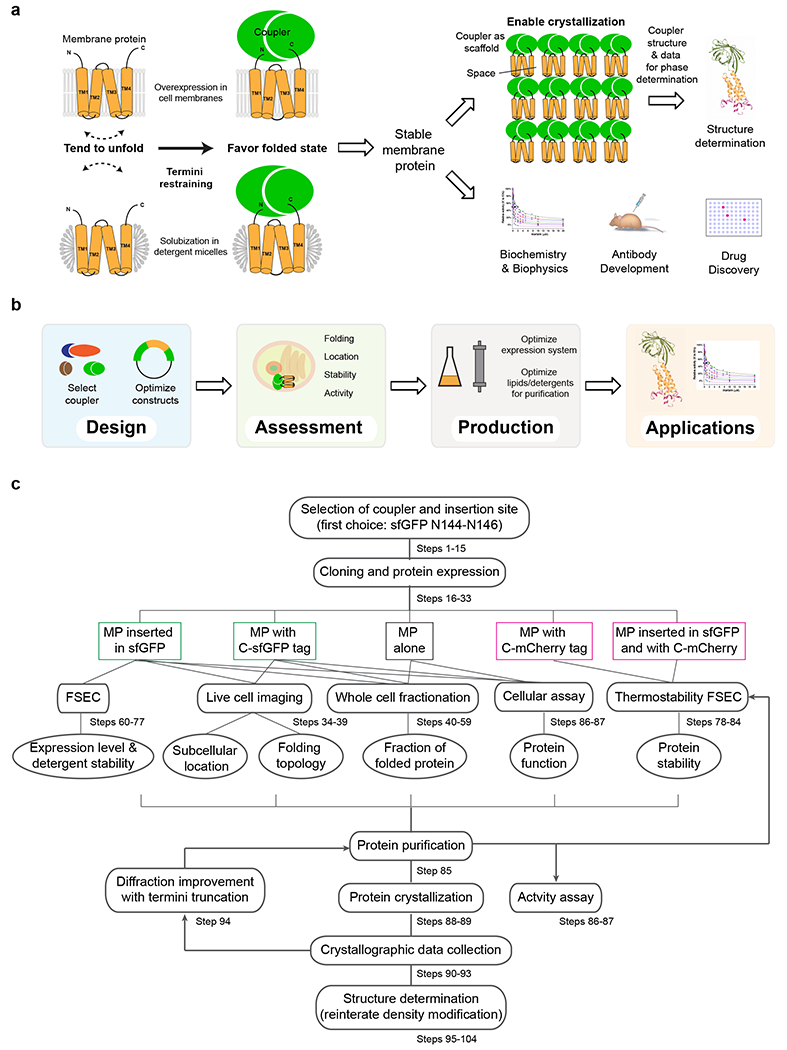
a, Membrane proteins (orange) are prone to unfold during overexpression in cells and after solubilization in detergents. The unfolding involves large motions of TMs (dashed arrow). To restrict such motions and stabilize the folded state, a self-assembling coupler protein (green) is fused to the flexible N and C termini of the membrane protein (orange) to provide a mild restraint. For structure determination, the coupler serves as a crystallization scaffold that promotes regular crystal packing, and increases the diffraction mass by lowering the relative space between non-contacting membrane regions, which is occupied by solvent and lipid/detergent. Existing structure and diffraction data of the coupler are used for solving the phase problem. Furthermore, stabilized membrane proteins are used in many biochemical and biophysical studies and pharmaceutical applications. b, The protocol consists of following stages: design and generation of restrained constructs, assessment of protein quality, large-scale expression and purification, and applications. c, Detailed workflow of the termini restraining protocol. MP: membrane protein.
