Fig. 2. |. Quality assessment of restrained membrane proteins.
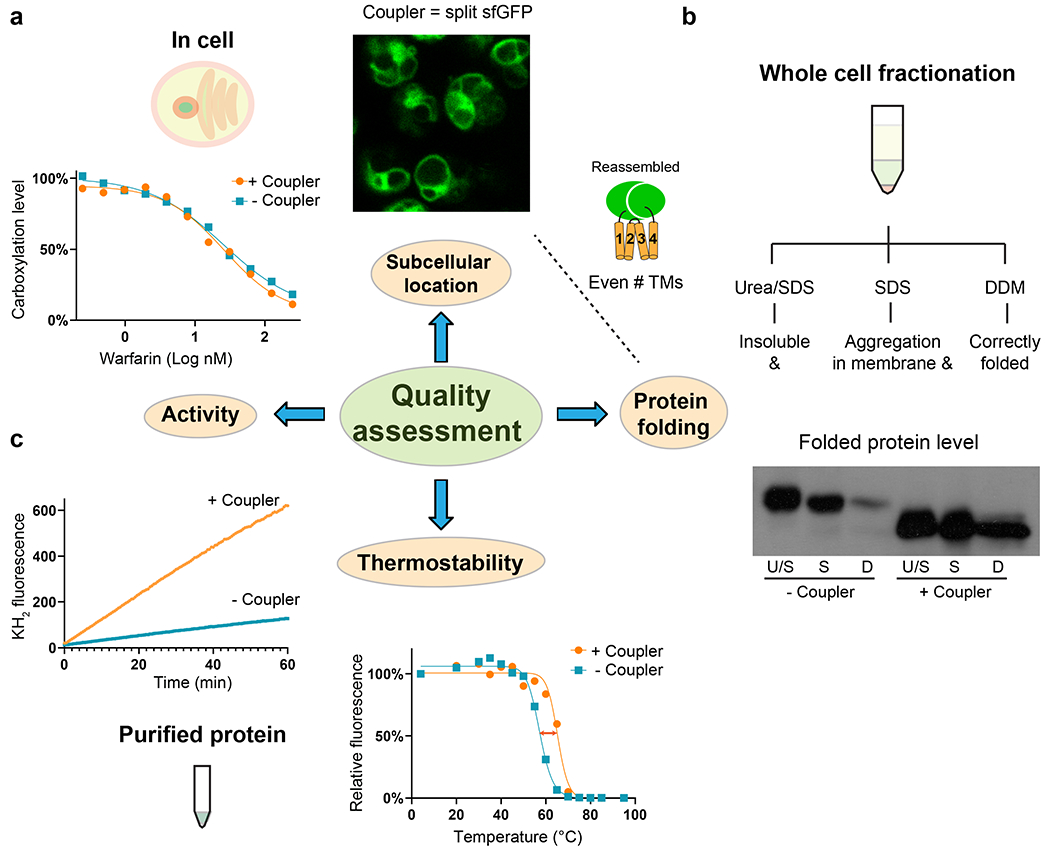
a, Cellular assays using sfGFP-restrained VKORL protein as an example. Left, VKORL is an integral membrane enzyme whose activity and inhibition by warfarin can be readily analyzed with a cellular γ-carboxylation assay. Right, Fluorescence imaging shows that restrained VKORL is correctly located in the endoplasmic reticulum membrane after overexpression in Pichia cells. Furthermore, the reconstituted GFP fluorescence indicates that restrained VKORL folds in the expected 4-TM (even numbered) folding topology. b, Whole-cell fractionation measurements of the folded protein level. The unrestrained and restrained VKORL are compared on Western blot. U/S: urea/SDS, S: SDS, D:DDM. Restrained VKORL shows a much larger DDM-extractable (well-folded) fraction. c, Verification of the activity of purified protein and measurement of improved thermostability. Left, The epoxide reductase activity of VKORL is monitored by increased fluorescence of the reaction product, vitamin K hydroquinone (KH2). Right, FSEC measurements show increased Tm with termini restraining.
