Figure 4. Lentivirus production and titration.
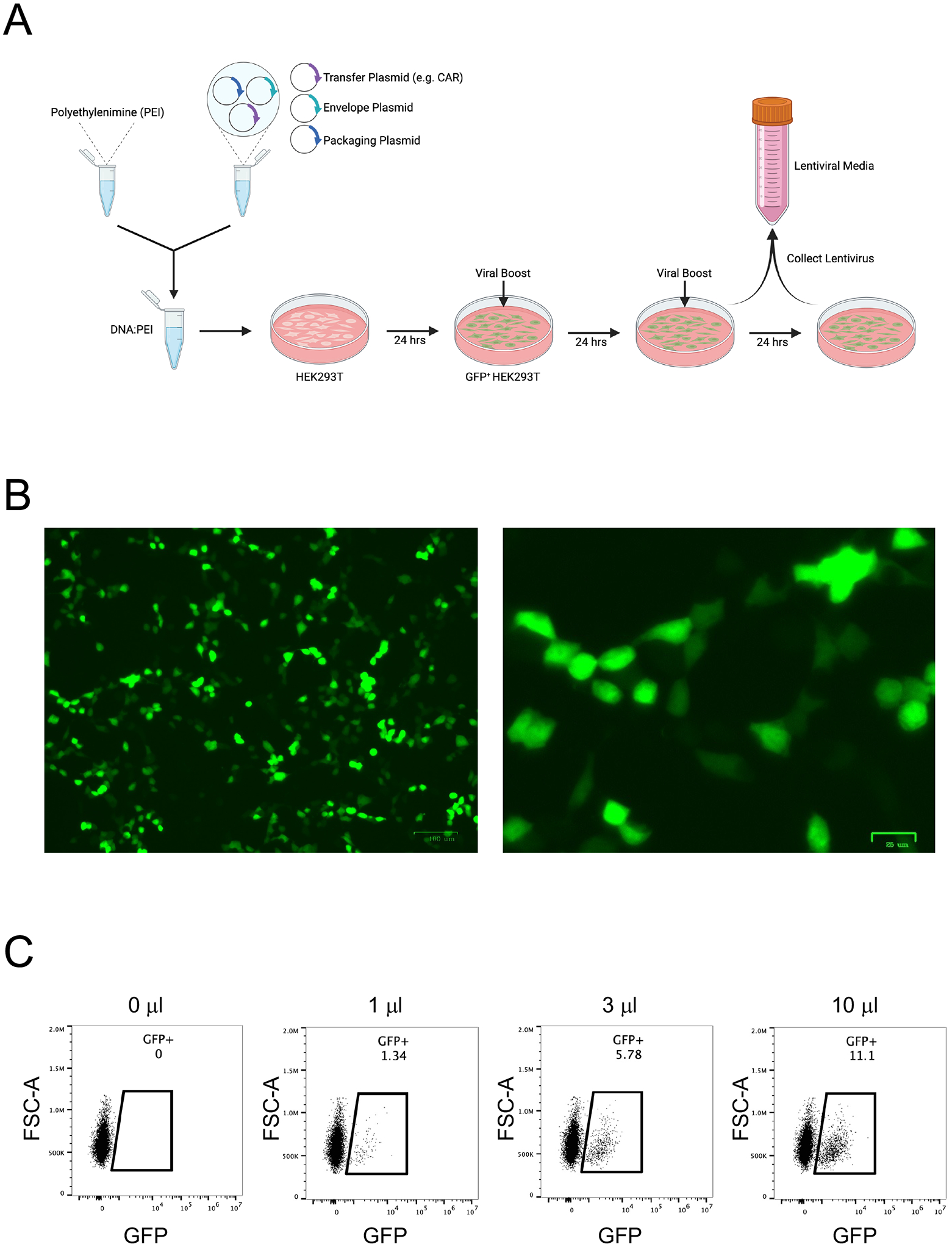
A. The workflow for lentivirus production involves transfecting chloroquine-treated HEK293T cells with transfer plasmid (CAR), envelope plasmid, and packaging plasmid using polyethylenimine (PEI). One day later, the medium is replaced with fresh medium containing ViralBoost. On the third and fourth days, lentivirus-containing medium is collected into conical tubes and stored at 4°C. B. GFP expression by HEK293T cells 18h after transfection with CD19CAR-2A-GFP accompanied by envelope plasmid and packaging plasmid, imaged at 5X (left) and 20X (right) magnification. C. Titration of CD19CAR-2A-GFP lentivirus particle-containing medium in Jurkat cells.
