Abstract
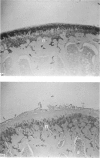
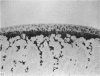
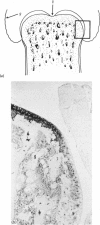
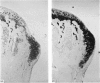
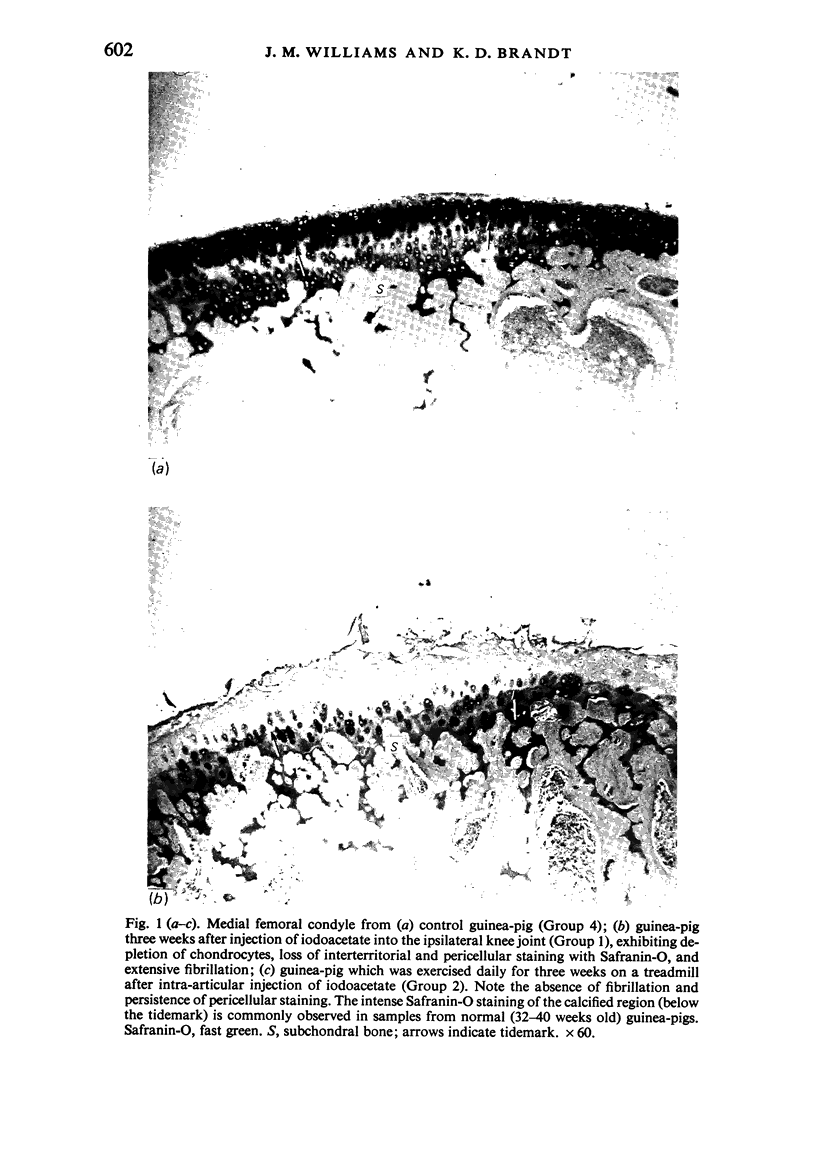
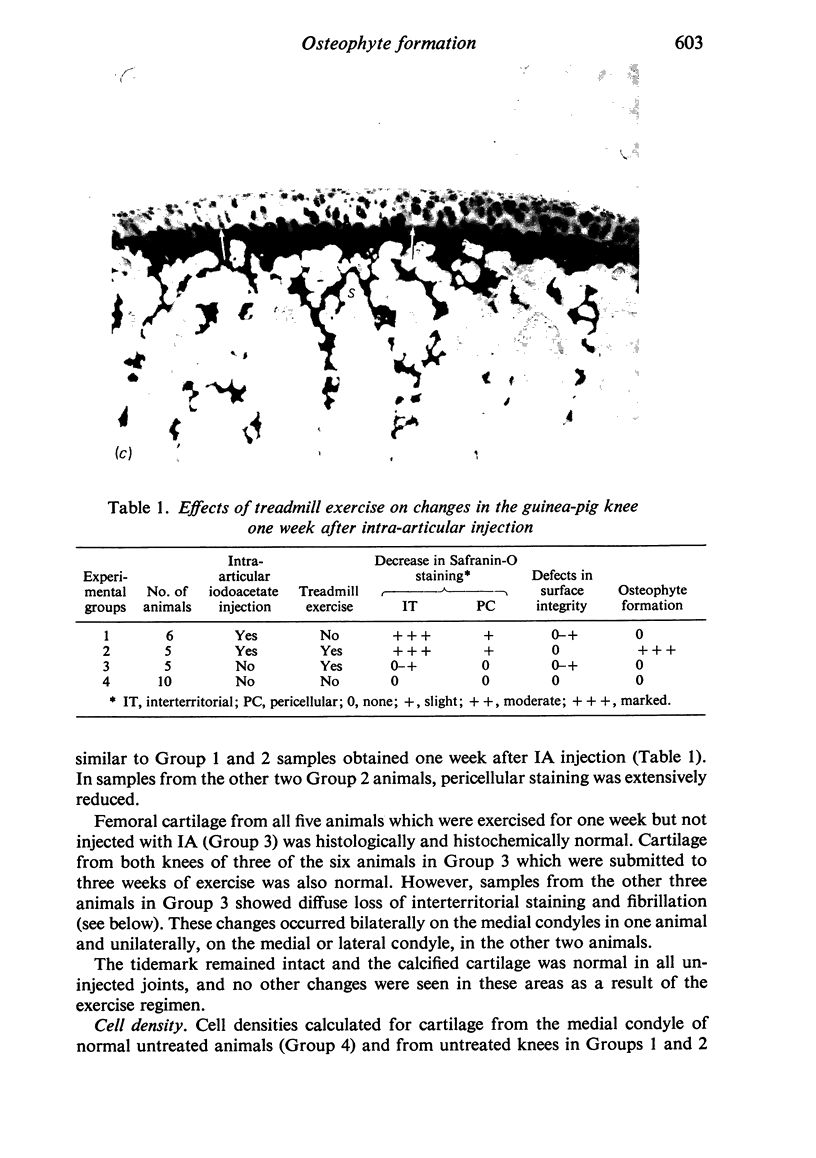
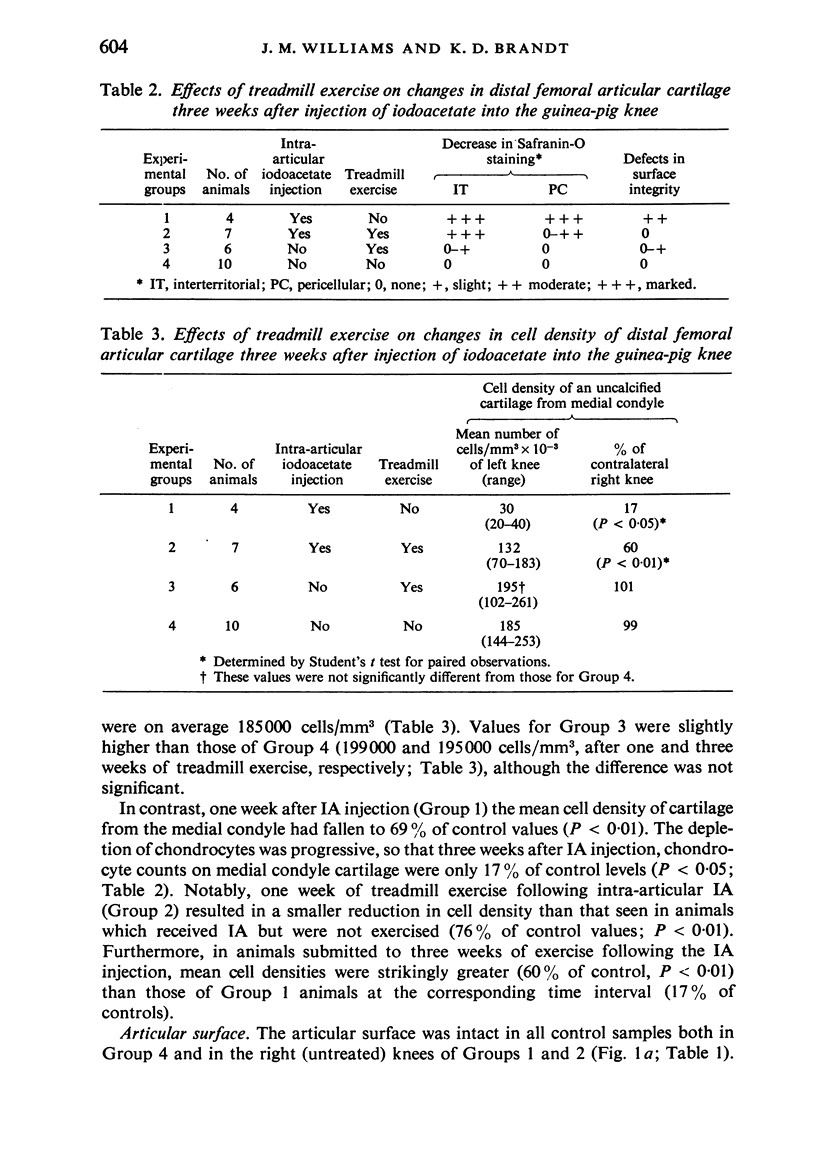
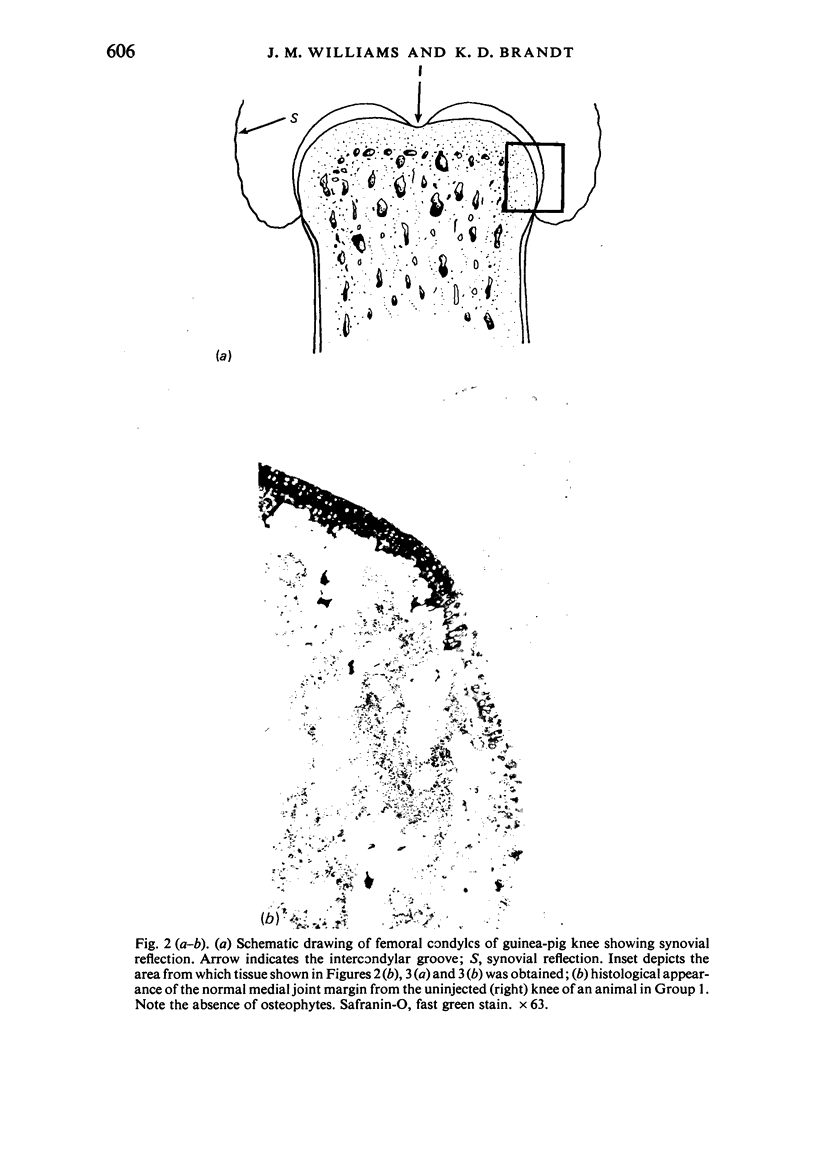
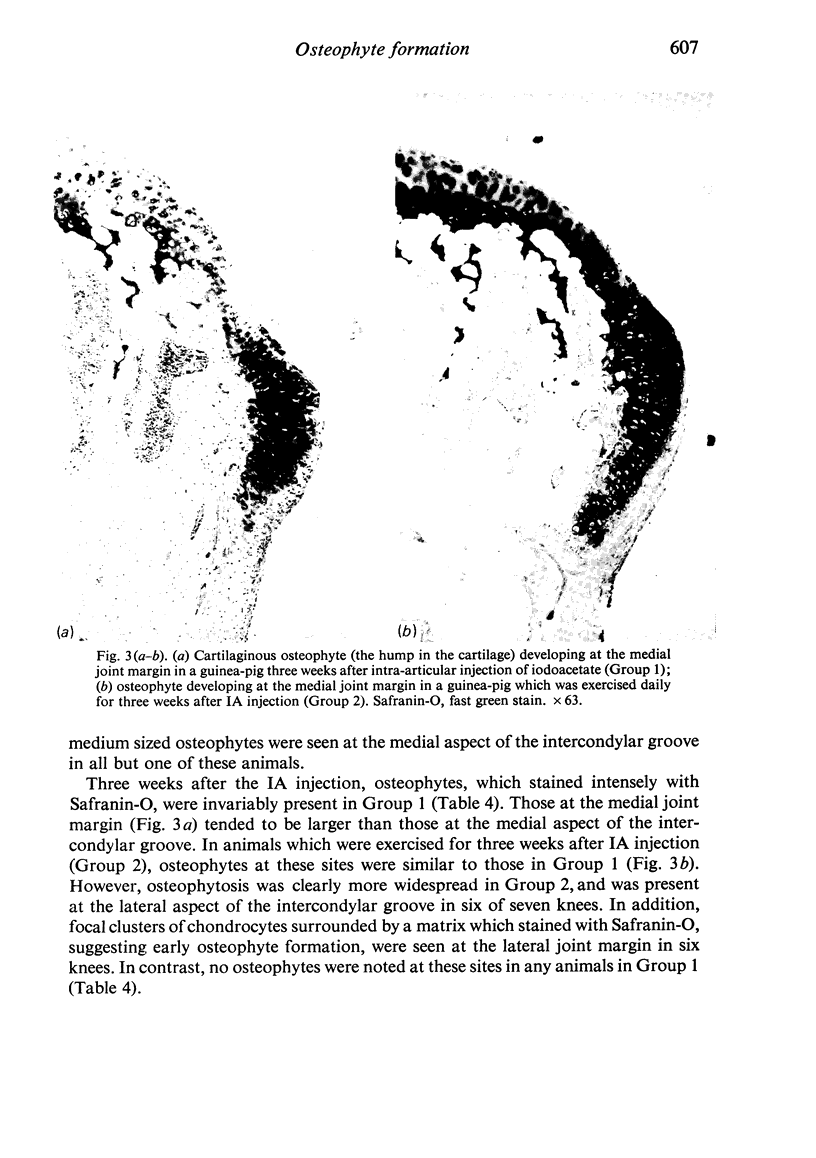
The present study shows that a treadmill exercise regimen imposed on guinea-pigs whose articular cartilage has been damaged by intra-articular injection of IA reduces chondrocyte depletion, results in an increase in pericellular Safranin-O staining around surviving chondrocytes, and prevents fibrillation of the articular surface. The data suggest that exercise protected, or facilitated recovery of, chondrocytes subjected to chemical injury, and that the surviving cells then synthesised a matrix which was sufficiently normal to withstand impulsive joint loading. On the other hand, the exercise regimen accelerated osteophyte formation, and led to formation of osteophytes in sites at which they did not develop in animals which received intra-articular IA but which were not exercised.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHRISMAN O. D., FESSEL J. M., SOUTHWICK W. O. EXPERIMENTAL PRODUCTION OF SYNOVITIS AND MARGINAL ARTICULAR EXOSTOSES IN THE KNEE JOINTS OF DOGS. Yale J Biol Med. 1965 Apr;37:409–412. [PMC free article] [PubMed] [Google Scholar]
- Danielsson L., Hernborg J. Morbidity and mortality of osteoarthritis of the knee (gonarthrosis) in Malmö, Sweden. Clin Orthop Relat Res. 1970 Mar-Apr;69:224–226. [PubMed] [Google Scholar]
- Gilbertson E. M. Development of periarticular osteophytes in experimentally induced osteoarthritis in the dog. A study using microradiographic, microangiographic, and fluorescent bone-labelling techniques. Ann Rheum Dis. 1975 Feb;34(1):12–25. doi: 10.1136/ard.34.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMRE C. J., YEAGER V. L. Influence of denervated muscles on exostoses of rats fed a sweet-pea diet. AMA Arch Pathol. 1958 Feb;65(2):215–227. [PubMed] [Google Scholar]
- HAMRE C. J., YEAGER V. L. Influence of muscle section on exostoses of lathyric rats. AMA Arch Pathol. 1957 Oct;64(4):426–433. [PubMed] [Google Scholar]
- Marshall J. L., Olsson S. E. Instability of the knee. A long-term experimental study in dogs. J Bone Joint Surg Am. 1971 Dec;53(8):1561–1570. [PubMed] [Google Scholar]
- Meachim G. Light microscopy of Indian ink preparations of fibrillated cartilage. Ann Rheum Dis. 1972 Nov;31(6):457–464. doi: 10.1136/ard.31.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Immobilization of the knee prevents osteoarthritis after anterior cruciate ligament transection. Arthritis Rheum. 1982 Oct;25(10):1201–1208. doi: 10.1002/art.1780251009. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Colyer R. A., Brandt K. D. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 1980 Mar;23(3):325–334. doi: 10.1002/art.1780230310. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971 Jan;53(1):69–82. [PubMed] [Google Scholar]
- SILVERSTEIN E., SOKOLOFF L. Natural history of degenerative joint disease in small laboratory animals. 5. Osteoarthritis in guinea pigs. Arthritis Rheum. 1958 Feb;1(1):82–86. doi: 10.1002/art.1780010112. [DOI] [PubMed] [Google Scholar]
- Salter R. B., Simmonds D. F., Malcolm B. W., Rumble E. J., MacMichael D., Clements N. D. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980 Dec;62(8):1232–1251. [PubMed] [Google Scholar]
- Simon W. H., Richardson S., Herman W., Parsons J. R., Lane J. Long-term effects of chondrocyte death on rabbit articular cartilage in vivo. J Bone Joint Surg Am. 1976 Jun;58(4):517–526. [PubMed] [Google Scholar]
- Stockwell R. A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971 Sep;109(Pt 3):411–421. [PMC free article] [PubMed] [Google Scholar]
- Telhag H., Lindberg L. A method for inducing osteoarthritic changes in rabbits' knees. Clin Orthop Relat Res. 1972 Jul-Aug;86:214–223. doi: 10.1097/00003086-197207000-00033. [DOI] [PubMed] [Google Scholar]
- Videman T. The effect of running on the osteoarthritic joint: an experimental matched-pair study with rabbits. Rheumatol Rehabil. 1982 Feb;21(1):1–8. doi: 10.1093/rheumatology/21.1.1. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Brandt K. D. Immobilization ameliorates chemically-induced articular cartilage damage. Arthritis Rheum. 1984 Feb;27(2):208–216. doi: 10.1002/art.1780270213. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Brandt K. D. Temporary immobilisation facilitates repair of chemically induced articular cartilage injury. J Anat. 1984 May;138(Pt 3):435–446. [PMC free article] [PubMed] [Google Scholar]