Highlights
-
•
Recalcitrant warts affect immunocompetent and immunocompromised persons.
-
•
The optimal approach to refractory warts remains unclear.
-
•
Human papillomavirus vaccination is a promising therapy for recalcitrant warts.
Keywords: Human papillomavirus, Vaccine, Cutaneous warts, Recalcitrant
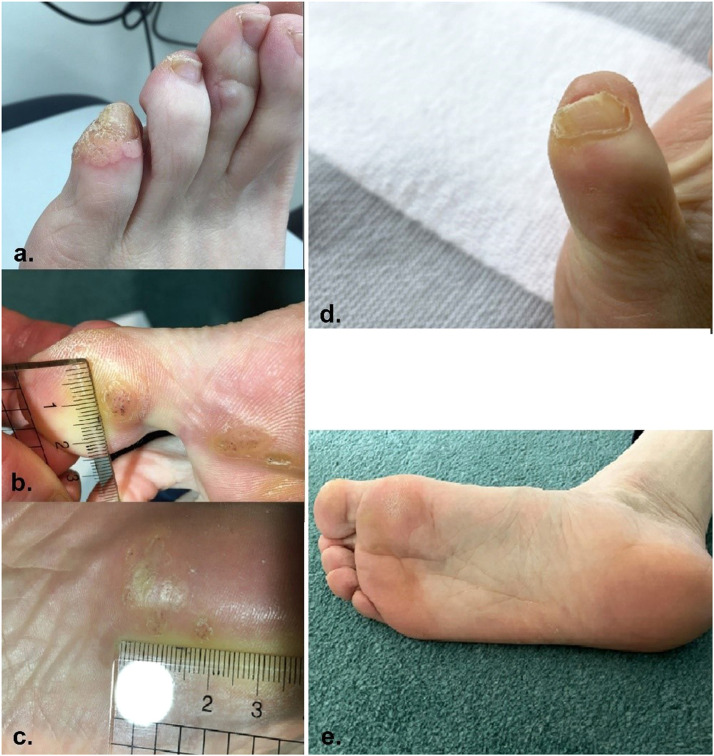
A 52-year-old woman with recurrent plantar warts since childhood was referred for immunologic evaluation. Topical treatments were only temporarily effective, with recurrence within months. Complete remission was noted 1 month after receiving the first dose of the 9-valent human papillomavirus (HPV) vaccine series (Figure 1).
Figure 1.
Cutaneous warts on left fifth toe and sole before (a, b, c) and after (d, e) administration of the first dose of the nine-valent human papillomavirus vaccine series.
Cutaneous warts are the most common manifestation of HPV skin infection, typically affecting children and young adults [1]. Diagnosis is clinical and based on presence of hyperkeratotic papules, thrombosed capillaries, and interruption of dermatoglyphics. Recalcitrant warts are characterized by failure to respond after five treatment courses and merit immunologic investigation [2].
Intact cell-mediated immunity is necessary for host defense against HPV. Recalcitrant warts, especially if extensive, affecting multiple sites or associated with lymphopenia, should raise suspicion for an underlying immunodeficiency. A detailed history should exclude secondary causes and focus on atopy, autoimmunity, or malignancy (i.e. common primary immunodeficiency associations). Notably, past infections associated with T-cell defects (e.g. Pneumocystis jirovecii pneumonia, severe Herpesviridae, or nontuberculous mycobacterial infections) should lead to targeted evaluation with quantitative immunoglobulins and lymphocyte subsets [3].
Resolution of cutaneous warts after HPV vaccination has been previously reported in retrospective studies. Yang et al. reported the use of the quadrivalent HPV vaccine in 30 patients with multiple warts, with 47% achieving complete remission and 17% partial response, and no severe adverse events were recorded [4]. In another retrospective study of 16 patients with recalcitrant warts, the use of the quadrivalent HPV vaccine was associated with 44% complete remission [5]. The recombinant nine-valent HPV vaccine for recalcitrant warts was evaluated in an open-label, single-arm study by Shin et al.; complete response rate was reported in 62.2% and partial response in 8.9% of a cohort of 45 patients [6]. Interestingly, as in our case, 37.5% of the responders showed clinical improvement after the first injection of the nine-valent HPV vaccine. Because HPV can evade host defenses even in immunocompetent persons, HPV vaccination merits further investigation for the management of recalcitrant warts.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors have contributed to the acquisition and analysis of the data, drafting and final approval of the article.
Ethical approval statement
All procedures were performed in compliance with relevant laws and institutional guidelines. Informed consent has been obtained for the publication of the medical imagery and case presentation.
References
- 1.Sterling JC, Gibbs S, Haque Hussain SS, Mohd Mustapa MF, Handfield-Jones SE, Hughes JR, et al. British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol. 2014;171:696–712. doi: 10.1111/bjd.13310. [DOI] [PubMed] [Google Scholar]
- 2.Leung L. Recalcitrant nongenital warts. Aust Fam Physician. 2011;40:40–42. [PubMed] [Google Scholar]
- 3.Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030–1048. doi: 10.1016/j.jaci.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang M-Y, Son J-H, Kim G-W, Kim HS, Ko HC, Kim MB, et al. Quadrivalent human papilloma virus vaccine for the treatment of multiple warts: a retrospective analysis of 30 patients. J Dermatolog Treat. 2019;30:405–409. doi: 10.1080/09546634.2018.1527006. [DOI] [PubMed] [Google Scholar]
- 5.Waldman A, Whiting D, Rani M, Alam M. HPV vaccine for treatment of recalcitrant cutaneous warts in adults: a retrospective cohort study. Dermatol Surg. 2019;45:1739–1741. doi: 10.1097/DSS.0000000000001867. [DOI] [PubMed] [Google Scholar]
- 6.Shin J-O, Son J-H, Lee J, Kim HS, Ko HC, Kim BS, et al. Nonavalent human papilloma virus vaccine for the treatment of multiple recalcitrant warts: an open-label study. J Am Acad Dermatol. 2022;86:940–941. doi: 10.1016/j.jaad.2021.03.074. [DOI] [PubMed] [Google Scholar]