Figure 1. The production of ROS in ferroptosis.
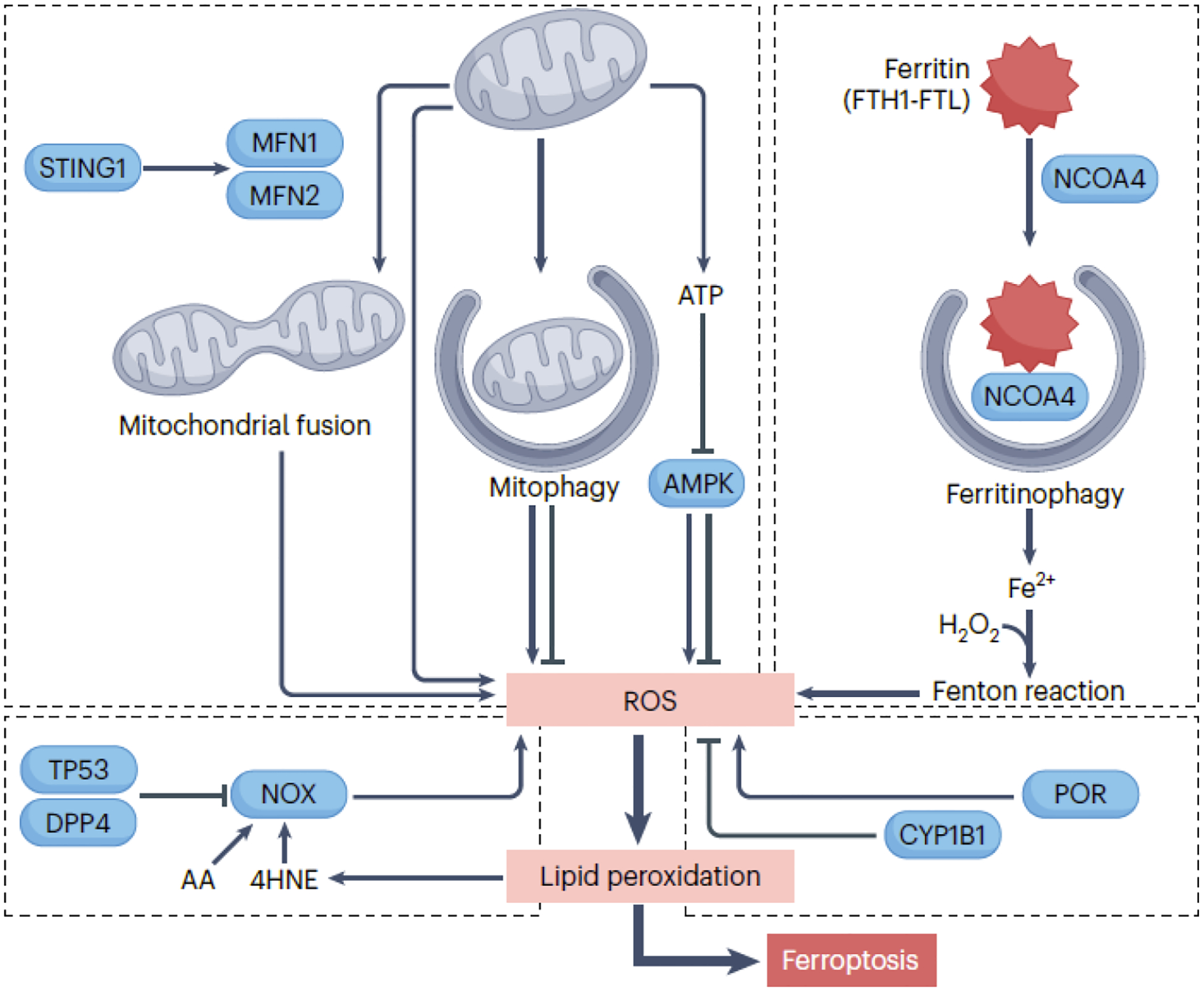
The initiation of ferroptosis requires an oxidative environment, facilitated by diverse sources of ROS. Mitochondrial ROS, primarily generated through the electron transport chain, can trigger ferroptosis in specific conditions. Mitophagy, involved in removing damaged mitochondria, has a dual role in promoting or inhibiting ferroptosis, while mitochondrial fusion increases cellular sensitivity to ferroptosis. Activation of the mitochondrial STING1 (stimulator of interferon response cGAMP interactor 1) may promote mitochondrial fusion, leading to ROS production implicated in ferroptosis. Mitochondrial energy stress activates AMPK, which can promote or inhibit ferroptosis by phosphorylating different substrates. NOX (NADPH oxidase) enzymes in cell membranes play a crucial role in generating ROS in ferroptosis. TP53 inhibits NOX-mediated ferroptosis by binding to DPP4 (dipeptidyl peptidase 4), while arachidonic acid (AA) and 4HNE enhance NOX1 activity to promote ROS production. POR (cytochrome p450 oxidoreductase) promotes ROS production and ferroptosis, whereas CYP1B1 (cytochrome P450 family 1 subfamily B member 1) inhibits ferroptosis. Ferritinophagy involves the degradation of the iron storage protein ferritin, releasing Fe2+ that triggers ROS production through the Fenton reaction.
