Figure 1.
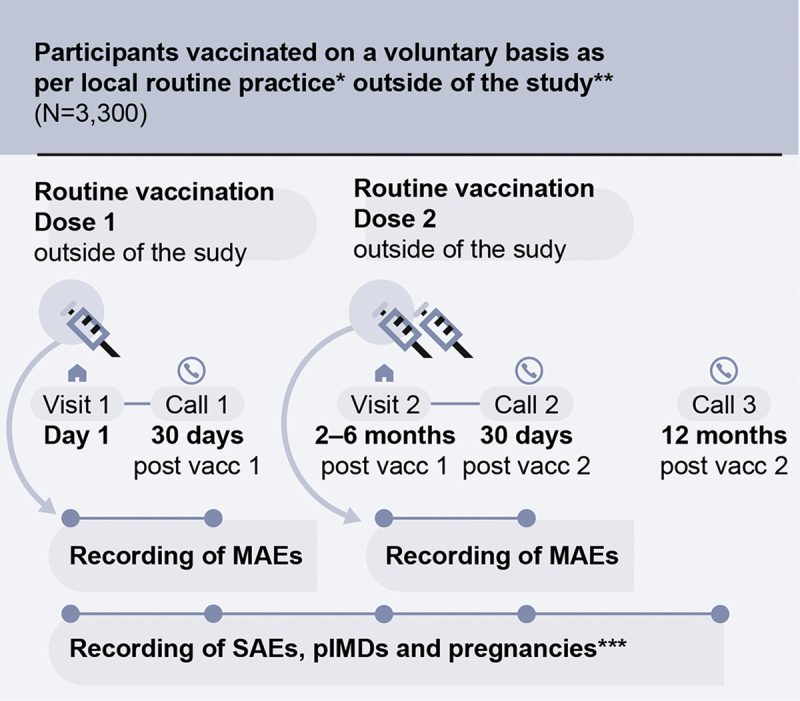
Study design.
* Local routine practice: two doses of the adjuvanted recombinant zoster vaccine (RZV) administered intramuscularly according to the local prescribing information (i.e., dose 2 administered 2–6 months post-dose 1); ** Outside of the study means that vaccinations were not study procedures (i.e., this was a non-interventional study). The participants’ decision to receive RZV was “outside of the study,” i.e., independent of their decision to participate in this study; *** Any exposure to RZV during pregnancy (any stage of gestation) needed to be reported to the study sponsor; MAEs, medically attended adverse events; N, number of participants; SAEs, serious adverse events; pIMDs, potential immune-mediated disorders; vacc, vaccine dose.
