Fig. 6.
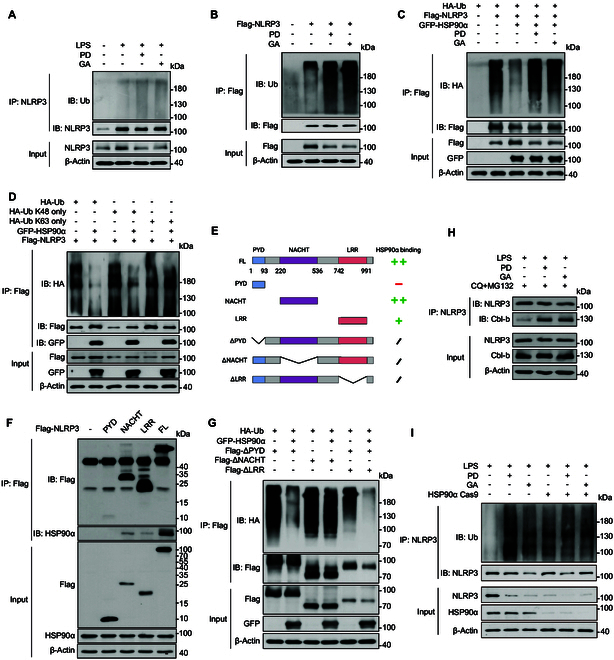
HSP90α inhibition promoted the ubiquitination of NLRP3 mediated by Cbl-b. (A) BMDMs treated with 10 μM polydatin or GA were stimulated with 100 ng/ml LPS for 3 h, or (B) HEK293T cells transfected with flag-NLRP3 were treated with 10 μM polydatin or GA. NLRP3 ubiquitination level was analyzed by immunoblot. (C) HEK293T cells were transfected with various combinations (above lanes) of plasmids encoding flag-NLRP3, GFP-HSP90α, and HA-Ub, and immunoblot analysis of NLRP3 ubiquitination (detected by anti-HA antibody) in cell lysates immunoprecipitated with anti-flag agarose. (D) HEK293T cells were transfected with various combinations (above lanes) of plasmids encoding flag-NLRP3, GFP-HSP90α and HA-Ub, HA-Ub K48 only, or K63 only. Immunoblot analysis of NLRP3 ubiquitination (detected by anti-HA antibody) in cell lysates immunoprecipitated with anti-flag agarose. (E) Schematic representation of NLRP3 WT and deletion mutants. (F) HEK293T cells were transfected with various combinations (above lanes) of plasmids encoding NLRP3 truncations as indicated. Immunoblot analysis of interaction between HSP90α and NLRP3 truncations in lysates immunoprecipitated with anti-flag agarose. (G) HEK293T cells were transfected with various combinations (above lanes) of plasmids encoding HA-Ub, GFP-HSP90α, and NLRP3 truncations as indicated. Immunoblot analysis of ubiquitination of NLRP3 truncations (detected by anti-HA antibody) in cell lysates immunoprecipitated with anti-flag agarose. (H) BMDMs treated with CQ and MG132 were added 10 μM polydatin or GA and then exposed to 100 ng/ml LPS for 3 h. An endogenous IP with NLRP3 antibody was performed. (I) BMDMs infected with HSP90α Cas9 lentivirus were treated with 10 μM polydatin or GA, followed by 100 ng/ml LPS for 3 h. NLRP3 ubiquitination level was analyzed by immunoblot. Data are representative of 3 independent experiments.
