Abstract
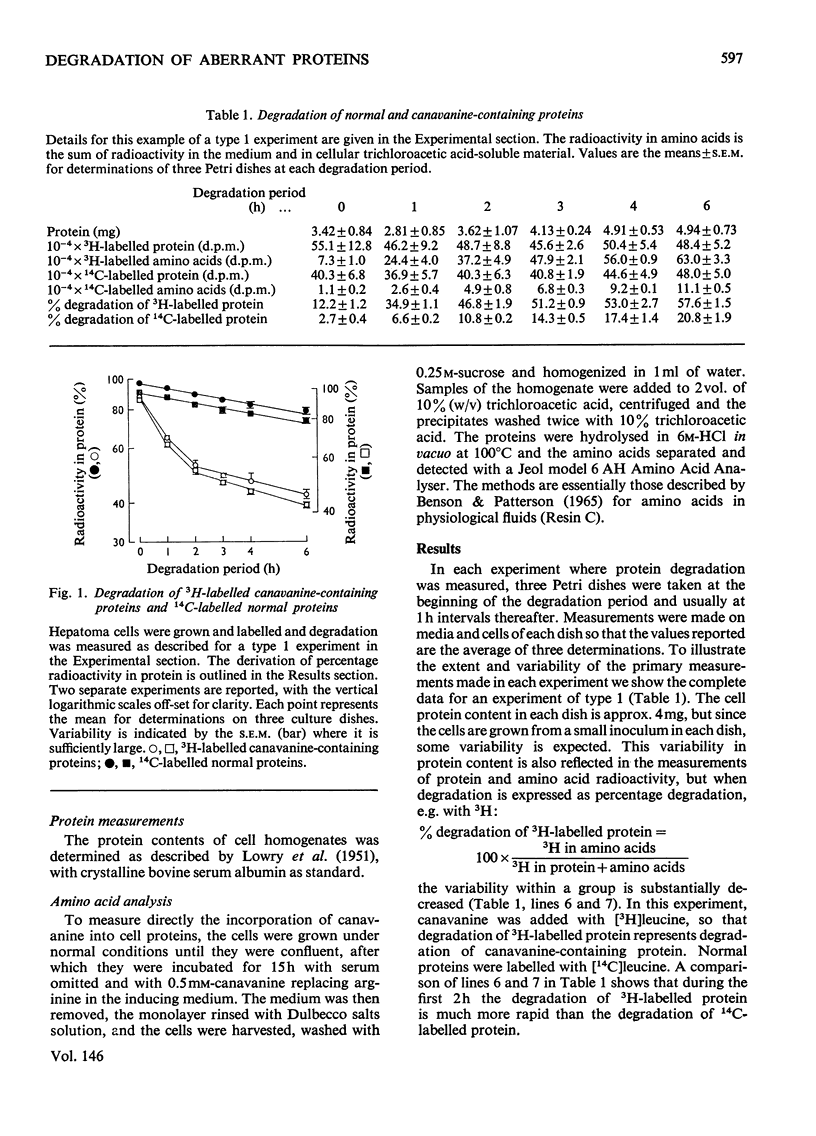
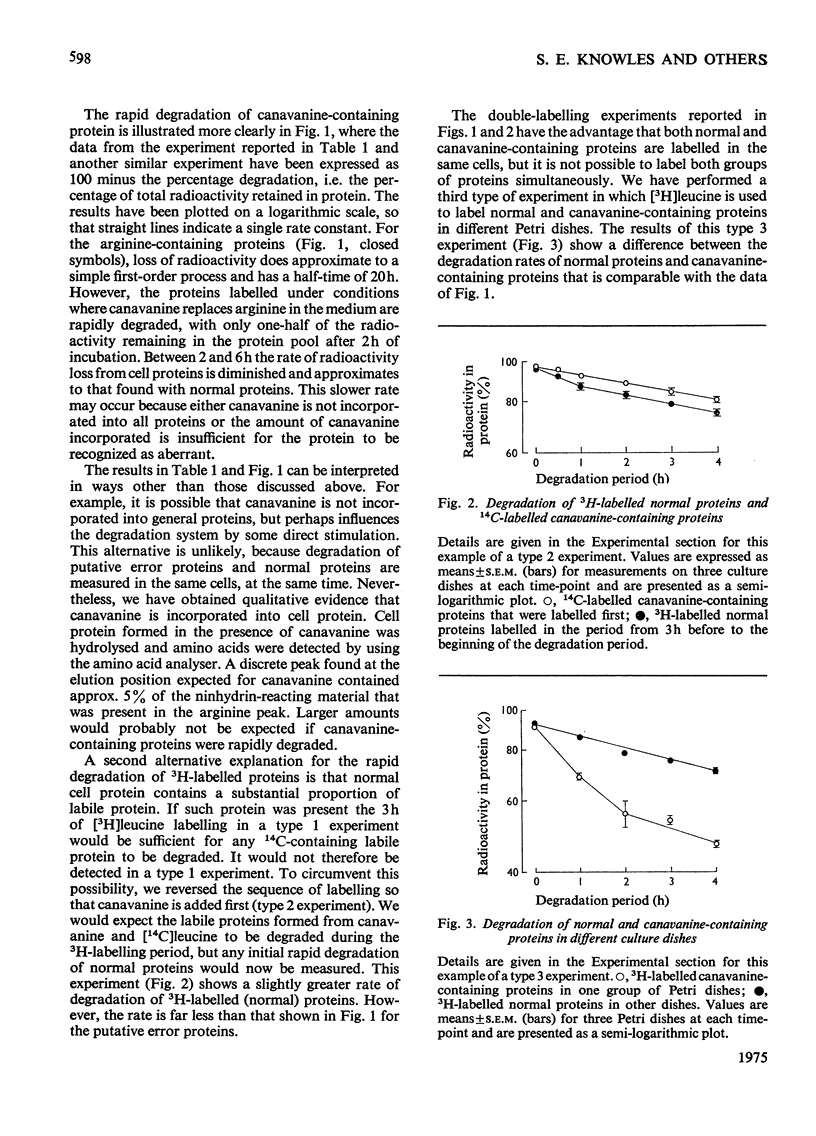
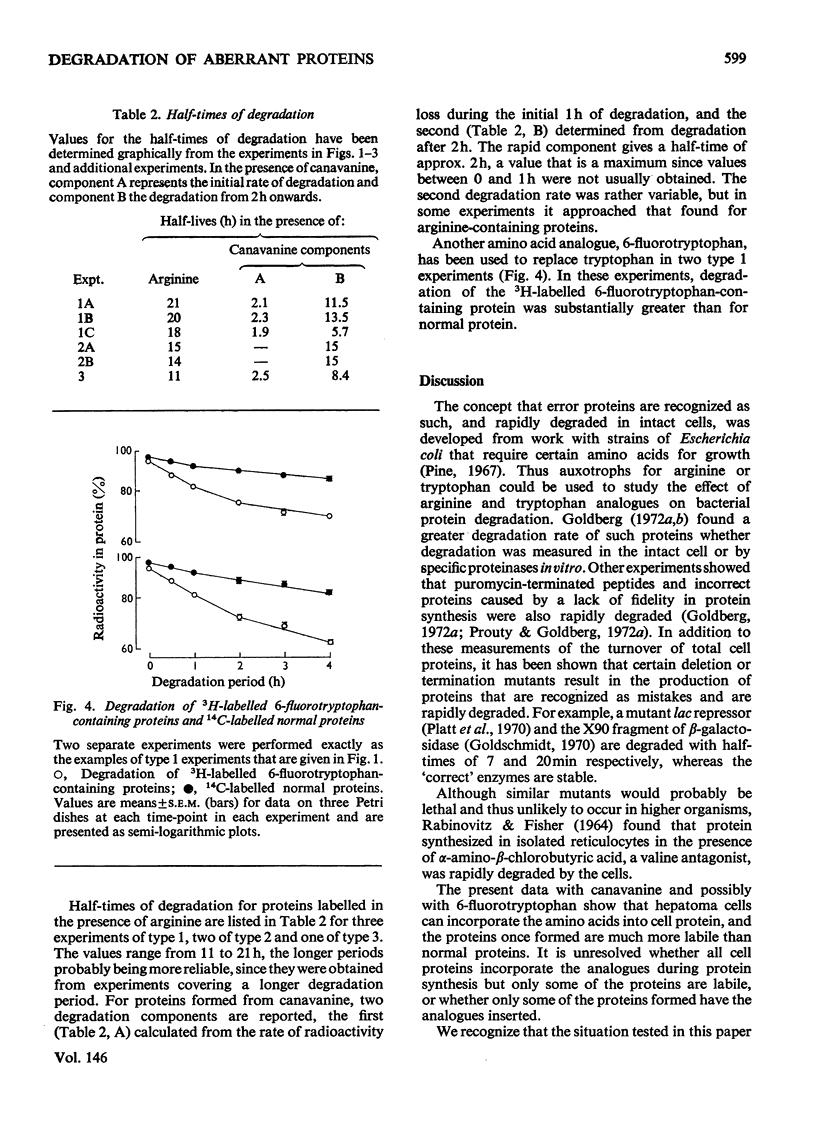
1. Reuber H35 hepatoma cells incorporate the arginine analogue canavanine into cell protein when arginine is omitted from the incubation medium. 2. By labelling arginine-containing proteins with (14-C)leucine and then canavanine-containing proteins with (3-H)leucine in the same cells, it is possible to measure the degradation of both types of protein during a subsequent 'chase' period. With this technique it has been shown that canavanine-containing proteins are degraded at a rate severalfold greater than normal proteins. Comparable results were found when 6-fluorotryptophan was used as an analogue to tryptophan. 3. Control experiments in which the labelling order was reversed or where the animo acid and its analogue were incubated in separate cell cultures support the conclusion that abberrant proteins are rapidly degraded in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J. Aging effects on the liver aldolase of rabbits. Biochem J. 1974 May;140(2):341–343. doi: 10.1042/bj1400341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. V., Jr, Patterson J. A. Accelerated chromatographic analysis of amino acids commonly found in physiological fluids on a spherical resin of specific design. Anal Biochem. 1965 Nov;13(2):265–280. doi: 10.1016/0003-2697(65)90196-x. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Gershon H., Gershon D. Inactive enzyme molecules in aging mice: liver aldolase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):909–913. doi: 10.1073/pnas.70.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Correlation between rates of degradation of bacterial proteins in vivo and their sensitivity to proteases. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2640–2644. doi: 10.1073/pnas.69.9.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- Holliday R., Tarrant G. M. Altered enzymes in ageing human fibroblasts. Nature. 1972 Jul 7;238(5358):26–30. doi: 10.1038/238026a0. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Ballard F. J. Synthesis and degradation of phosphoenolpyruvate carboxylase in rat liver and adipose tissue. Changes during a starvation-re-feeding cycle. Biochem J. 1973 Jun;134(2):445–453. doi: 10.1042/bj1340445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. W., Kenney F. T. Regulation of tyrosine aminotransferase in rat liver. XI. Studies on the relationship of enzyme stability to enzyme turnover in cultured hepatoma cells. J Biol Chem. 1973 Jul 10;248(13):4528–4531. [PubMed] [Google Scholar]
- Knowles S. E., Gunn J. M., Reshef L., Hanson R. W., Ballard F. J. Properties of phosphoenolpyruvate carboxykinase (guanosine triphosphate) synthesized in hepatoma cells in the presence of amino acid analogues. Biochem J. 1975 Mar;146(3):585–593. doi: 10.1042/bj1460585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C., MORSE P. A., Jr, POTTER V. R. HEPATOMAS IN TISSUE CULTURE COMPARED WITH ADAPTING LIVER IN VIVO. Natl Cancer Inst Monogr. 1964 Apr;13:229–245. [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Miller J. H., Weber K. In vivo degradation of mutant lac repressor. Nature. 1970 Dec 19;228(5277):1154–1156. doi: 10.1038/2281154a0. [DOI] [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Effects of protease inhibitors on protein breakdown in Escherichia coli. J Biol Chem. 1972 May 25;247(10):3341–3352. [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Fate of abnormal proteins in E. coli accumulation in intracellular granules before catabolism. Nat New Biol. 1972 Nov 29;240(100):147–150. doi: 10.1038/newbio240147a0. [DOI] [PubMed] [Google Scholar]
- RABINOVITZ M., FISHER J. M. CHARACTERISTICS OF THE INHIBITION OF HEMOGLOBIN SYNTHESIS IN RABBIT RETICULOCYTES BY THREO-ALPHA-AMINO-BETA-CHLOROBUTYRIC ACID. Biochim Biophys Acta. 1964 Oct 16;91:313–322. doi: 10.1016/0926-6550(64)90255-5. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]


