Abstract
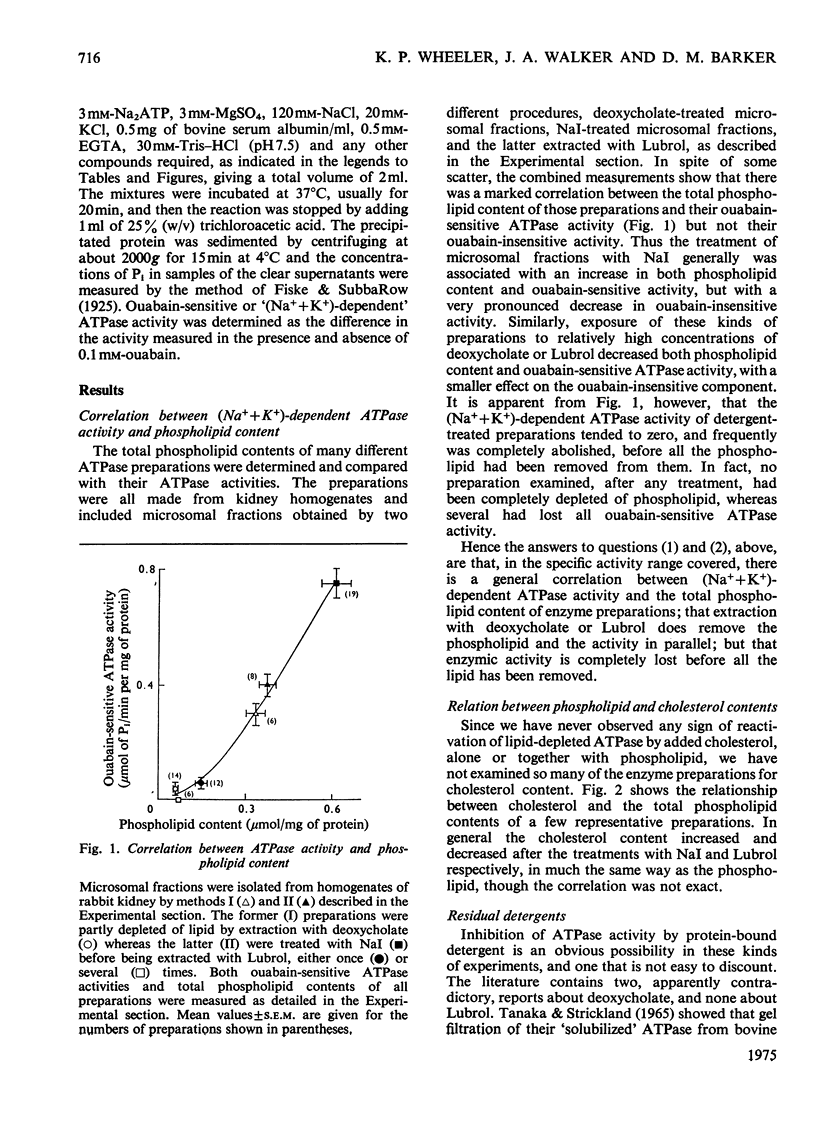
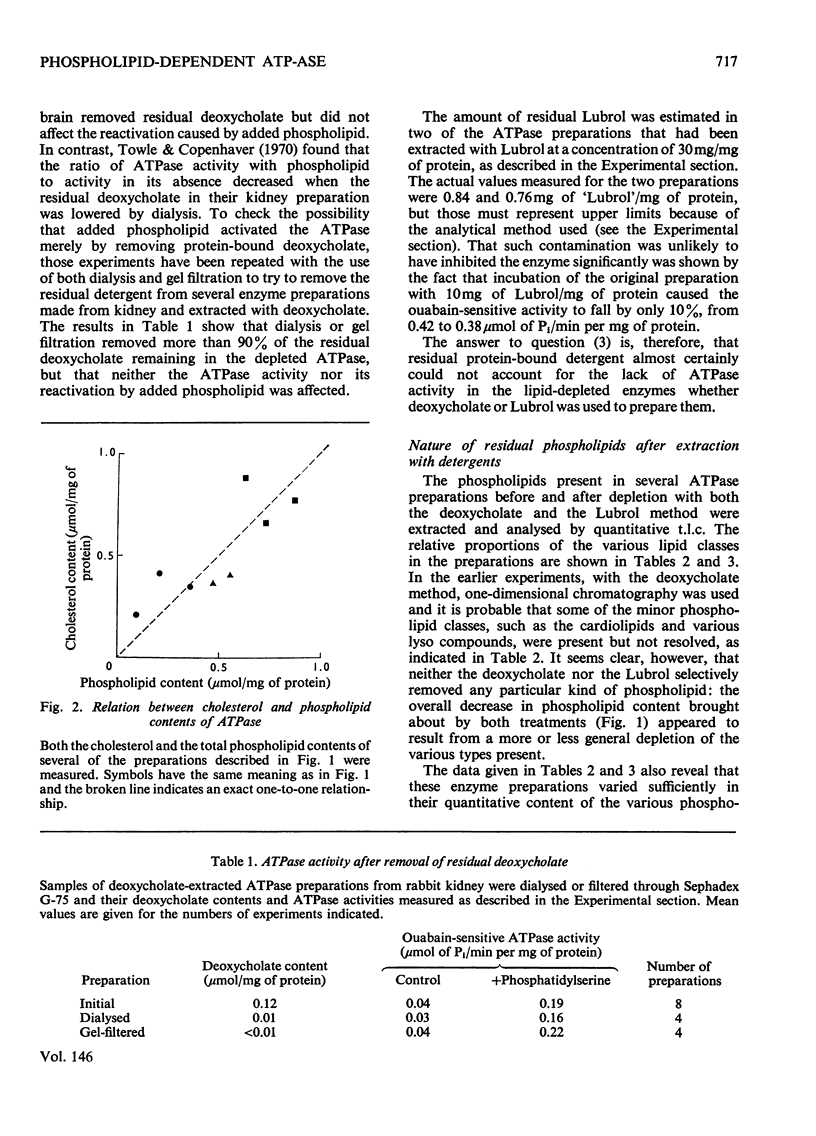
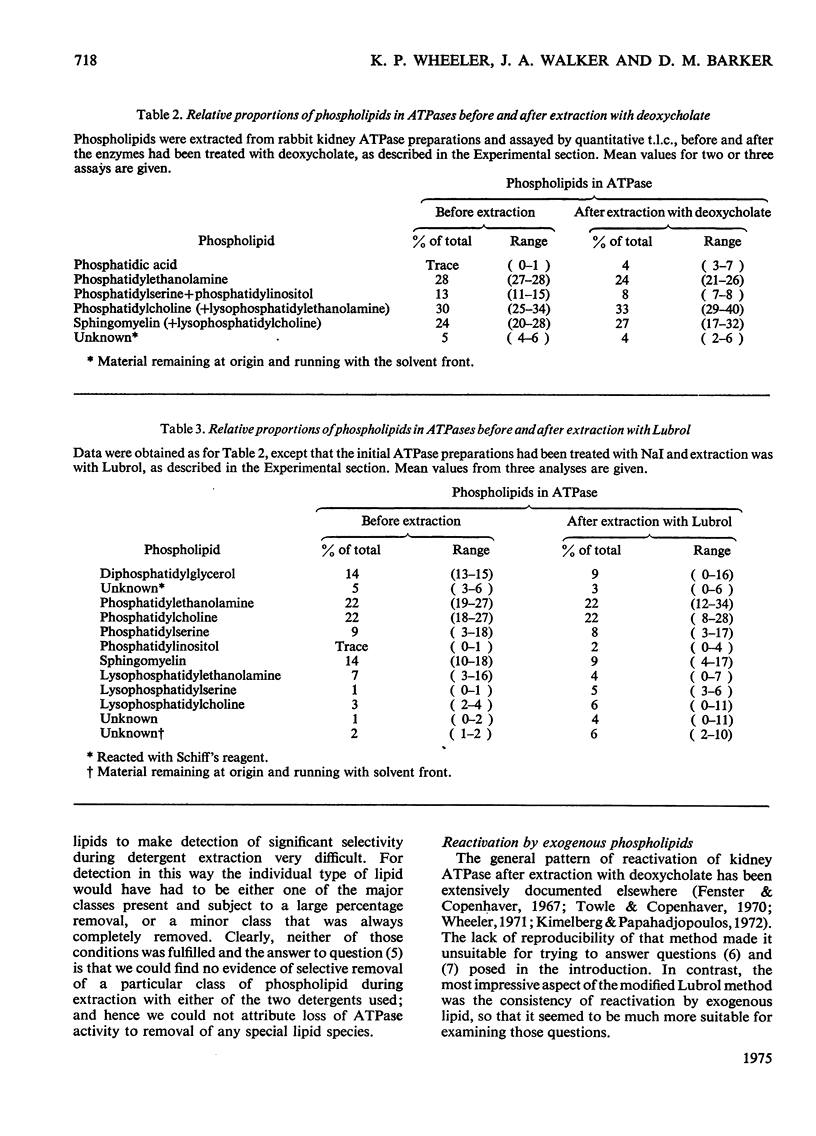
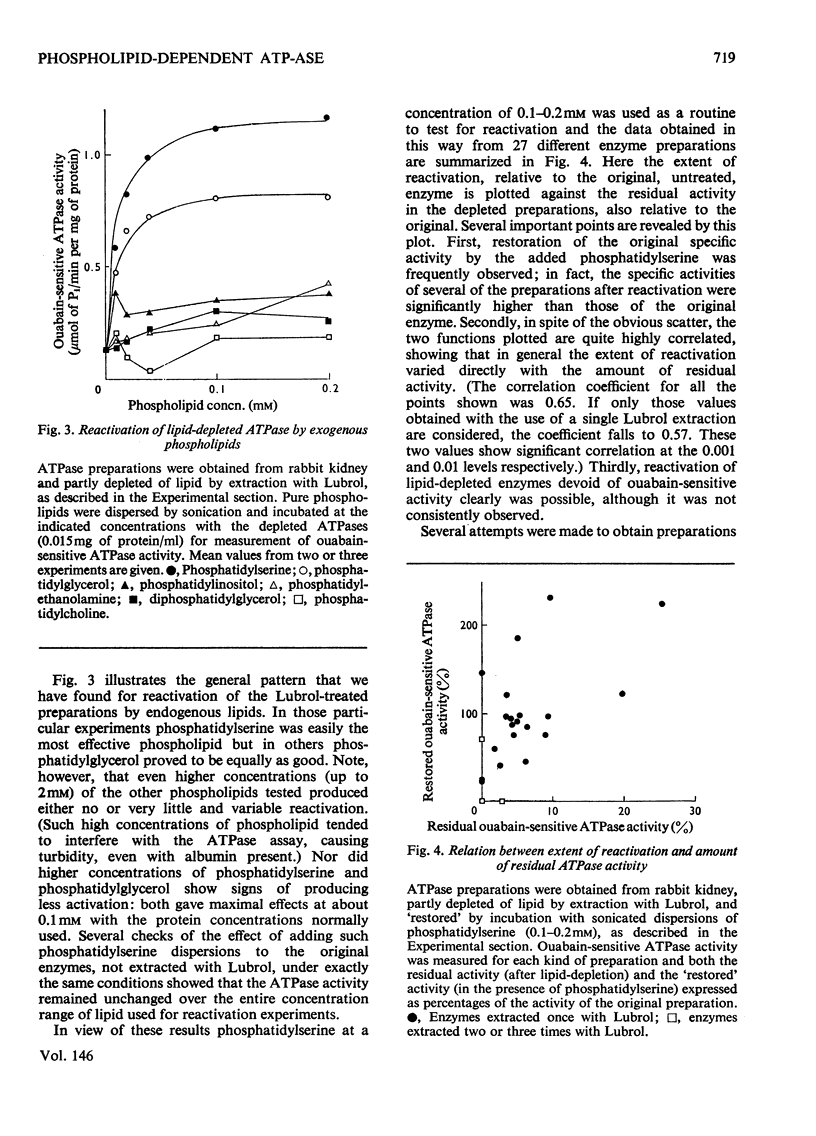
The dependence of the (Na-++K-+)-dependent ATPase (adenosine triphosphatase) (EC 3.6.1.3) on lipid has been examined in a number of different ways, with the use of various preparations from kidney tissue. The main findings were as follows. (1) The ATPase activities of the preparations examined were closely correlated with their total phospholipid content. (2) Extraction of the ATPase with deoxycholate or Lubrol W, combined with suitable salt-fractionation and washing procedures, removed phospholipid, cholesterol and enzymic activity in parallel; but activity was completely lost before all lipid had been removed. (3) The loss of activity could not be attributed to inhibition by residual detergent. (4) No selective removal of any particular phospholipid class by detergent could be detected. (5) Consistent reactivation of the Lubrol-extracted enzymes was obtained by adding dispersions of exogenous phospholipid, but only some, bearing a net negative charge, such as phosphatidylserine and phosphatidylglycerol, were effective. (6) The degree of reactivation was correlated with the amount of residual activity remaining after lipid depletion. (7) Partial purification of the ATPase, giving a 50-fold increase in specific activity, was not accompanied by selective enhancement of any particular class of phospholipid. We conclude that although the ATPase is dependent on phospholipid, only the reactivation results provide evidence for specificity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fenster L. J., Copenhaver J. H., Jr Phosphatidyl serine requirement of (Na+-K+)-activated adenosine triphosphatase from rat kidney and brain. Biochim Biophys Acta. 1967 Apr 4;137(2):406–408. doi: 10.1016/0005-2760(67)90120-8. [DOI] [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The interrelationship of membrane and protein structure in the functioning of the (Na + = K + )-activated ATPase. Biochim Biophys Acta. 1972 Jun 20;266(3):613–624. doi: 10.1016/0006-3002(72)90005-4. [DOI] [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The role of lipid-phase transitions in the regulation of the (sodium + potassium) adenosine triphosphatase. Biochemistry. 1973 Jul 3;12(14):2635–2637. doi: 10.1021/bi00738a013. [DOI] [PubMed] [Google Scholar]
- Hokin L. E., Hexum T. D. Studies on the characterization of the sodium-potassium transport adenosine triphosphatase. Arch Biochem Biophys. 1972 Aug;151(2):453–463. doi: 10.1016/0003-9861(72)90521-8. [DOI] [PubMed] [Google Scholar]
- Kawai K., Nakao M., Nakao T., Fujita M. Purification and some properties of Na, K-transport ATPase. 3. Comparison of lipid and protein components on Na, K-ATPase preparations at various purification steps from pig brain. J Biochem. 1973 May;73(5):979–991. doi: 10.1093/oxfordjournals.jbchem.a130182. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Phospholipid requirements for (Na + + K + )-ATPase activity: head-group specificity and fatty acid fluidity. Biochim Biophys Acta. 1972 Sep 1;282(1):277–292. doi: 10.1016/0005-2736(72)90334-3. [DOI] [PubMed] [Google Scholar]
- MOSBACH E. H., KALINSKY H. J., HALPERN E., KENDALL F. E. Determination of deoxycholic and cholic acids in bile. Arch Biochem Biophys. 1954 Aug;51(2):402–410. doi: 10.1016/0003-9861(54)90495-6. [DOI] [PubMed] [Google Scholar]
- Nakao T., Tashima Y., Nagano K., Nakao M. Highly specific sodium-potassium-activated adenosine triphosphatase from various tissues of rabbit. Biochem Biophys Res Commun. 1965 Jun 9;19(6):755–758. doi: 10.1016/0006-291x(65)90323-2. [DOI] [PubMed] [Google Scholar]
- Palatini P., Dabbeni-Sala F., Bruni A. Reactivation of a phospholipid-depleted sodium, potassium-stimulated ATPase. Biochim Biophys Acta. 1972 Nov 2;288(2):413–422. doi: 10.1016/0005-2736(72)90262-3. [DOI] [PubMed] [Google Scholar]
- Roelofsen B., van Deenen L. L. Lipid requirement of membrane-bound ATPase. Studies on human erythrocyte ghosts. Eur J Biochem. 1973 Dec 3;40(1):245–257. doi: 10.1111/j.1432-1033.1973.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. Preparation from mammallian brain and kidney of the enzyme system involved in active transport of Na ions and K ions. Biochim Biophys Acta. 1962 Apr 9;58:314–325. doi: 10.1016/0006-3002(62)91015-6. [DOI] [PubMed] [Google Scholar]
- Tanaka R., Strickland K. P. Role of phospholipid in the activation of Na+, Ka+-activated adenosine triphosphatase of beef brain. Arch Biochem Biophys. 1965 Sep;111(3):583–592. doi: 10.1016/0003-9861(65)90239-0. [DOI] [PubMed] [Google Scholar]
- Towle D. W., Copenhaver J. H., Jr Partial purification of a soluble (Na+ + K+)--dependent ATPase from rabbit kidney. Biochim Biophys Acta. 1970 Mar 17;203(1):124–132. doi: 10.1016/0005-2736(70)90042-8. [DOI] [PubMed] [Google Scholar]
- Wheeler K. P., Whittam R. The involvement of phosphatidylserine in adenosine triphosphatase activity of the sodium pump. J Physiol. 1970 Apr;207(2):303–328. doi: 10.1113/jphysiol.1970.sp009063. [DOI] [PMC free article] [PubMed] [Google Scholar]


