Abstract
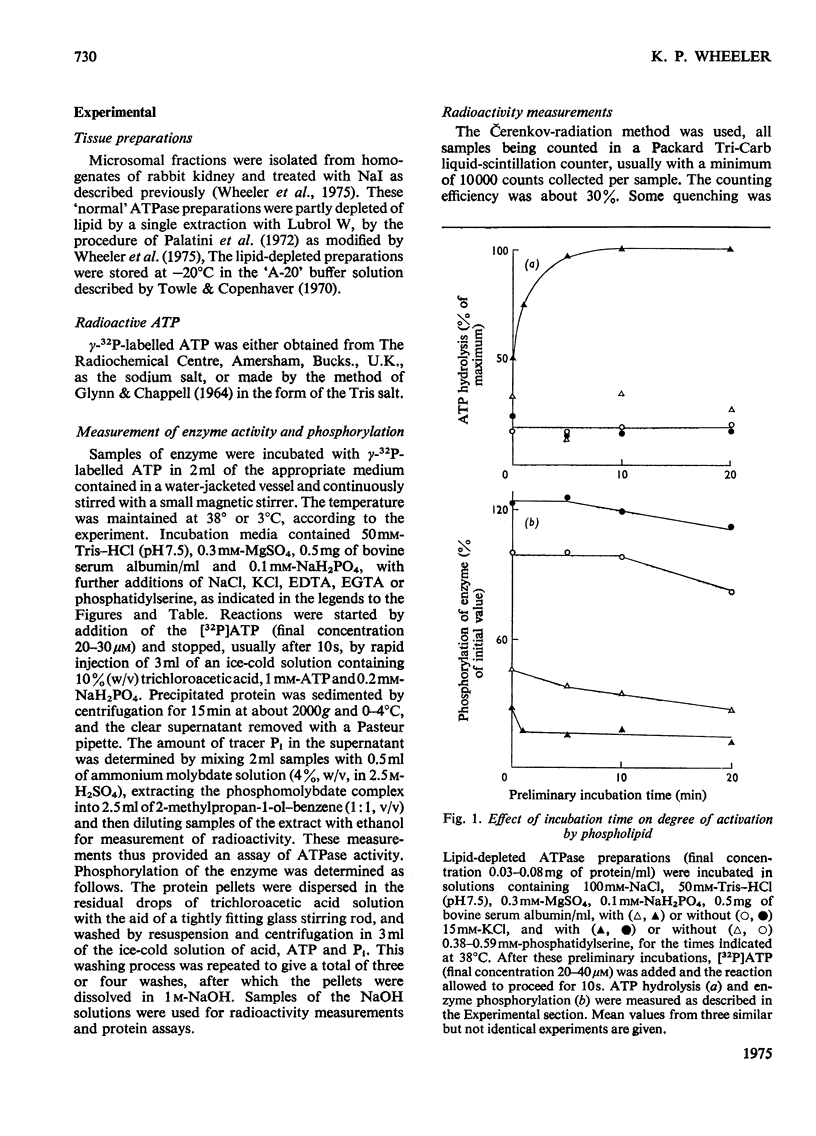
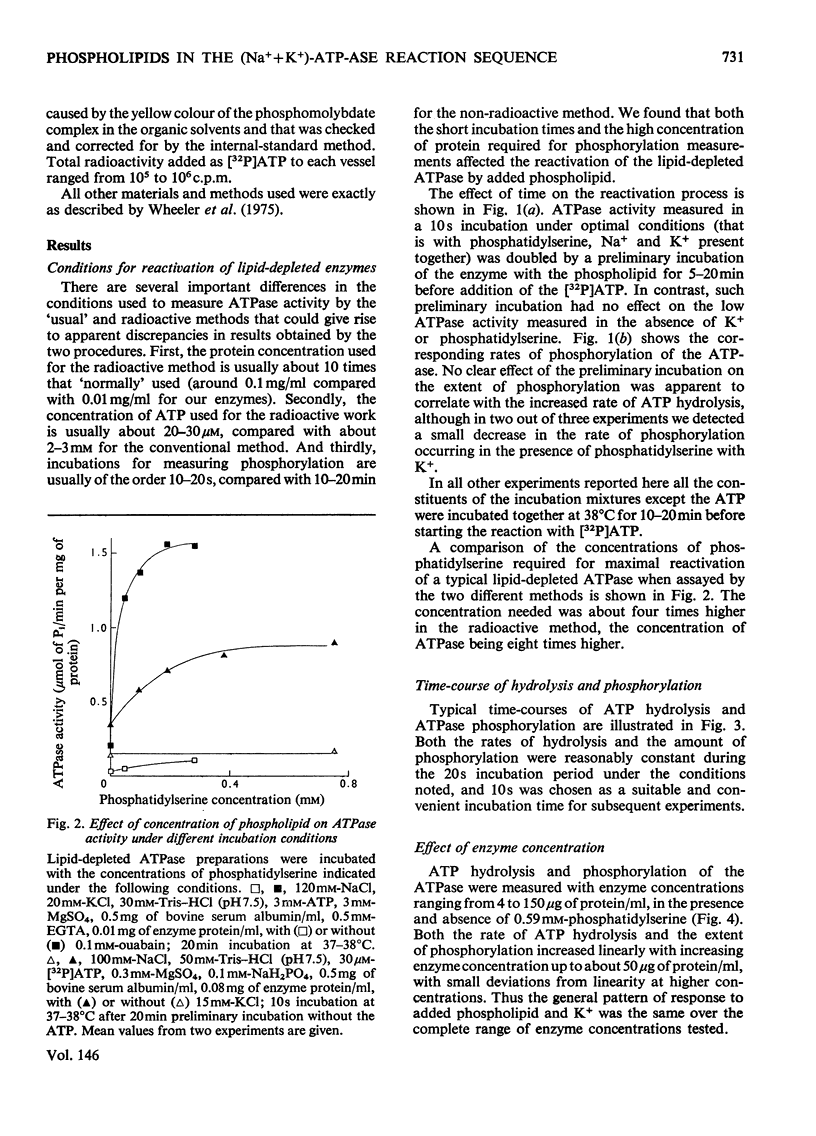
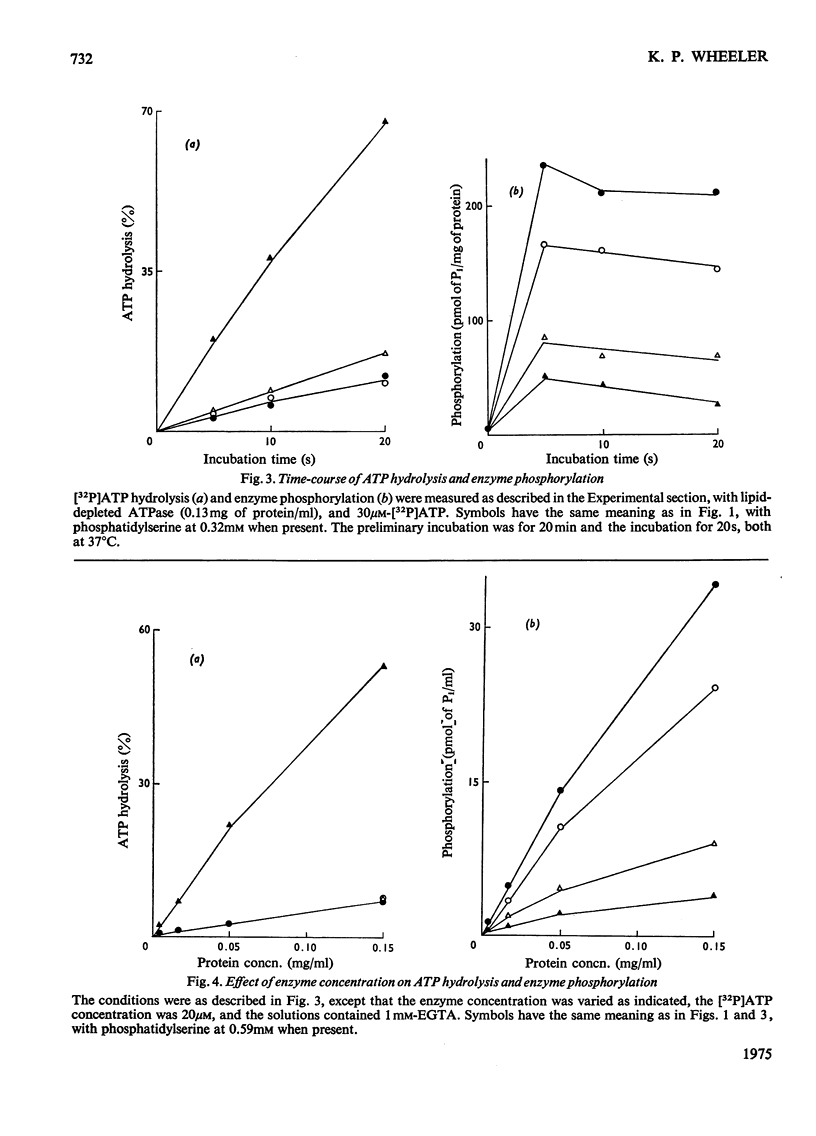
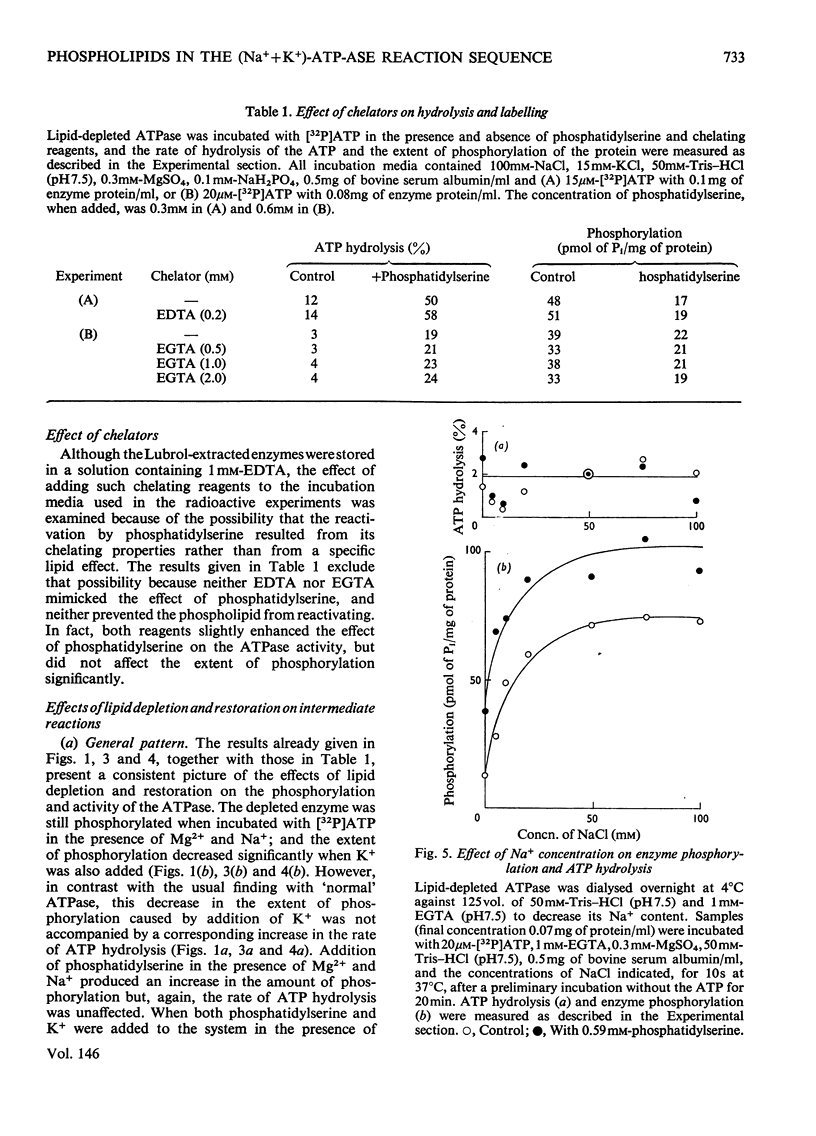
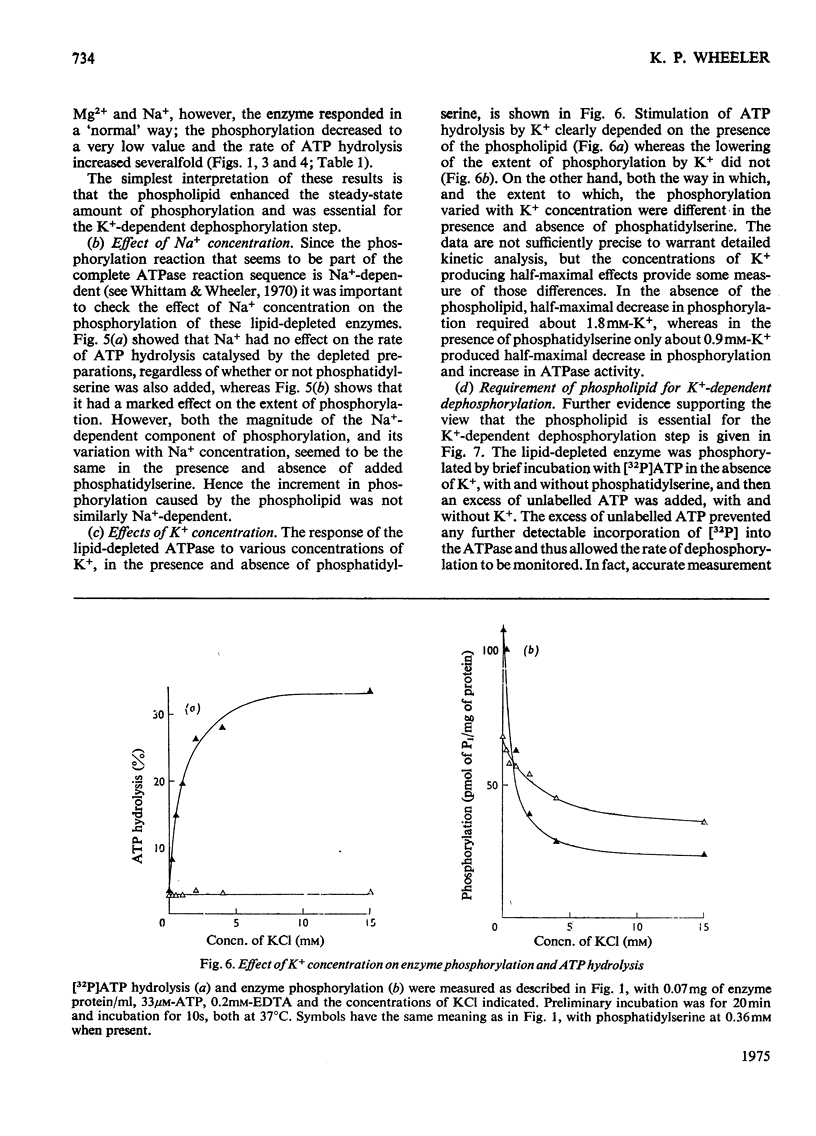
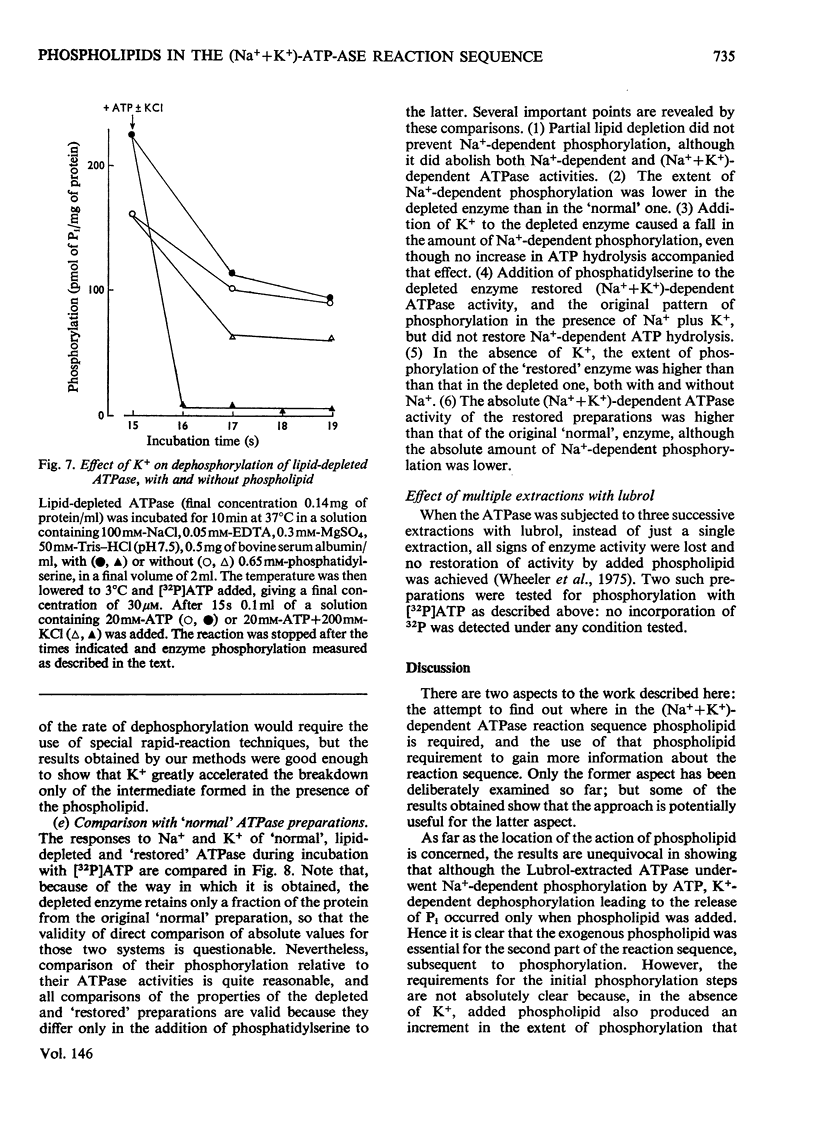
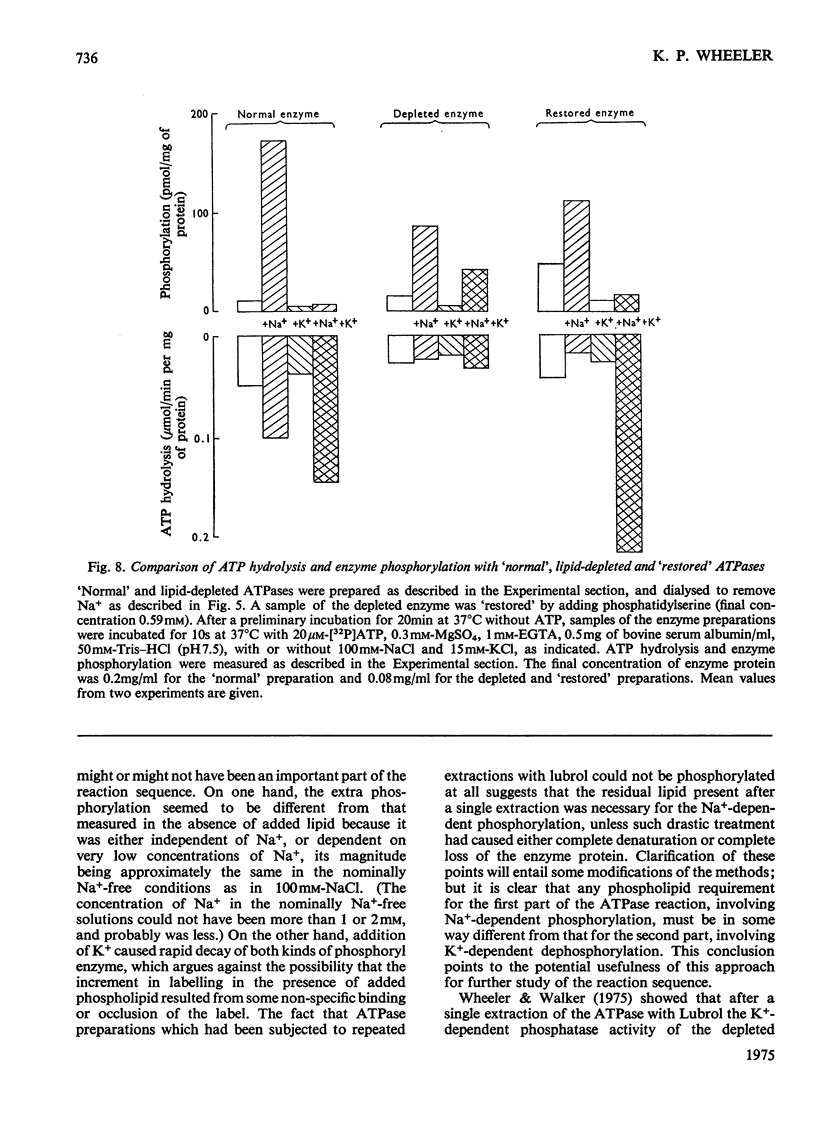
The phosphorylation and dephosphorylation steps of the (Na-++K-+)-dependent ATPase (adenosine triphosphatase) (EC 3.6.1.3) reaction have been compared in 'normal', lipid-depleted and 'restored' membrane ATPase preparations. Partial lipid depletion was achieved by a single extraction with Lubrol W, and 'restoration' by adding pure phosphatidylserine. Gamma-32-P-labelled ATP was used for phosphorylation. The main findings were as follows. (1) Partial lipid depletion decreased but did not prevent Na-+-dependent phosphorylation, although it virtually abolished both Na-+-dependent and (Na-++K-+)-dependent ATPase activities. (2) 'Restoration' with phosphatidylserine produced an increment in phosphorylation that was the same in the presence and absence of added Na-+. (3) K-+ decreased the extent of Na-+-dependent phosphorylation of the depleted enzyme without producing a corresponding release of Pi. (4) K-+ rapidly decreased the extent of phosphorylation of the 'restored' enzyme to near-background value, with a concomitant release of Pi. (5) Na-+-dependent ATP hydrolysis was not restored. (6) The turnover of the 'restored' enzyme seemed to be higher than that of the 'normal' enzyme. The reaction sequence is discussed in relation to these results and the fact that the depleted enzyme retained about 50% of K-+-dependent phosphatase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S. P., Wong S. M. Effect of potassium on sodium-dependent adenosine diphosphate-adenosine triphosphate exchange activity in kidney microsomes. J Biol Chem. 1972 Sep 10;247(17):5409–5413. [PubMed] [Google Scholar]
- Fukushima Y., Tonomura Y. Two kinds of high energy phosphorylated intermediate, with and without bound ADP, in the reaction of Na+K+dependent ATPase. J Biochem. 1973 Jul;74(1):135–142. doi: 10.1093/oxfordjournals.jbchem.a130216. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb P. S., Rodnight R. The role of bound potassium ions in the hydrolysis of low concentrations of adenosine triphosphate by preparations of membrane fragments from ox brain cerebral cortex. Biochem J. 1970 Nov;120(1):15–24. doi: 10.1042/bj1200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. S., Albers R. W. Sodium-potassium-activated adenosine triphosphatase. IX. The role of phospholipids. J Biol Chem. 1973 Feb 10;248(3):867–874. [PubMed] [Google Scholar]
- Palatini P., Dabbeni-Sala F., Bruni A. Reactivation of a phospholipid-depleted sodium, potassium-stimulated ATPase. Biochim Biophys Acta. 1972 Nov 2;288(2):413–422. doi: 10.1016/0005-2736(72)90262-3. [DOI] [PubMed] [Google Scholar]
- Post R. L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6530–6540. [PubMed] [Google Scholar]
- Roelofsen B., van Deenen L. L. Lipid requirement of membrane-bound ATPase. Studies on human erythrocyte ghosts. Eur J Biochem. 1973 Dec 3;40(1):245–257. doi: 10.1111/j.1432-1033.1973.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Siegel G. J., Goodwin B. Sodium-potassium-activated adenosine triphosphatase: potassium regulation of enzyme phosphorylation. Sodium-stimulated, potassium-inhibited uridine triphosphate hydrolysis. J Biol Chem. 1972 Jun 10;247(11):3630–3637. [PubMed] [Google Scholar]
- Stahl W. L. Ro le of phospholipids in the NA + ,K + -stimulated adenosine triphosphatase system of brain microsomes. Arch Biochem Biophys. 1973 Jan;154(1):56–67. doi: 10.1016/0003-9861(73)90034-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Tonomura Y. Inactivation of Na plus-K plus-dependent ATPase by phospholipase-treatment and its reactivation by phospholipids. J Biochem. 1971 Mar;69(3):543–557. [PubMed] [Google Scholar]
- Towle D. W., Copenhaver J. H., Jr Partial purification of a soluble (Na+ + K+)--dependent ATPase from rabbit kidney. Biochim Biophys Acta. 1970 Mar 17;203(1):124–132. doi: 10.1016/0005-2736(70)90042-8. [DOI] [PubMed] [Google Scholar]
- Wheeler K. P., Walker J. A., Barker D. M. Lipid requirement of the membrane sodium-plus-potassium ion-dependent adenosine triphosphatase system. Biochem J. 1975 Mar;146(3):713–722. doi: 10.1042/bj1460713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler K. P., Walker J. A. Differential effects of lipid depletion on membrane sodium-plus-potassium ion-dependent adenosine triphosphatase and potassium ion-dependent phosphatase. Biochem J. 1975 Mar;146(3):723–727. doi: 10.1042/bj1460723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Wheeler K. P. Transport across cell membranes. Annu Rev Physiol. 1970;32:21–60. doi: 10.1146/annurev.ph.32.030170.000321. [DOI] [PubMed] [Google Scholar]
- Winter C. G. Differential effects of digitonin on some enzyme activities of the sodium pump. Biochim Biophys Acta. 1972 Apr 14;266(1):135–143. doi: 10.1016/0005-2736(72)90129-0. [DOI] [PubMed] [Google Scholar]


